
Europe Blood Glucose Monitor Market
Europe Blood Glucose Monitor Market by Product Type (Self Blood Glucose Monitoring, Professional Point of Care), Application (Diabetes Management, Health & Wellness Monitoring), Test Site (Fingertip, Upper Arm), End User (Self/Homecare) - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
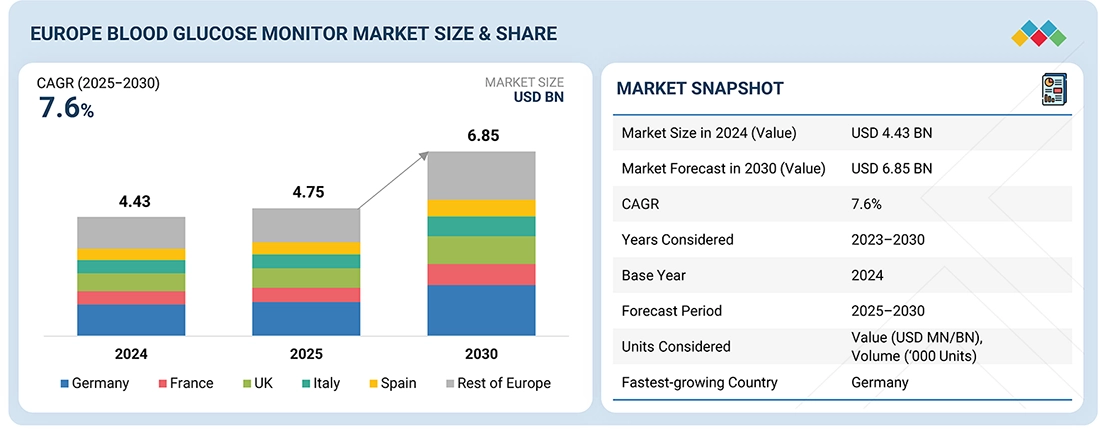
The Europe blood glucose monitor market is projected to reach 6.85 billion in 2030 from USD 4.75 billion in 2025, growing at a CAGR of 7.6% during the forecast period. The regional market is set for steady growth, due to the advancements in continuous glucose monitoring (CGM) and smart self-monitoring devices that improve accuracy, real-time data access, and ease of use. Rising diabetes prevalence, greater health awareness, and wider adoption of digital health solutions are further boosting the demand across Europe’s healthcare systems.
KEY TAKEAWAYS
-
BY COUNTRYBy country, Germany is likely to grow at a CAGR of 8.4% in the Europe blood glucose monitor market during the forecast period.
-
BY PRODUCT TYPEBased on product type, the self monitoring blood glucose systems segment held the maximum share of 42.1% in the Europe blood glucose monitor market in 2024.
-
BY APPLICATIONBased on application, the diabetes management segment is expected to grow at the highest CAGR of 7.7% during the forecast period.
-
BY TEST SITEBased on the test site, the fingertip segment contributed the largest share due to its convenience, fast results, and extensive usage among patients and healthcare professionals.
-
BY END USERBased on end user, the self/home care segment accounted for the largest share in the market.
-
COMPETITIVE LANDSCAPE- KEY PLAYERSAbbott Laboratories (US), DexCom, Inc. (US), and Medtronic (Ireland) were identified as some of the key players in the Europe blood glucose monitor market, given their substantial market share and product/service footprint.
-
COMPETITIVE LANDSCAPE- STARTUPSCompanies such as DiaMonTech AG and NovioSense B.V. have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
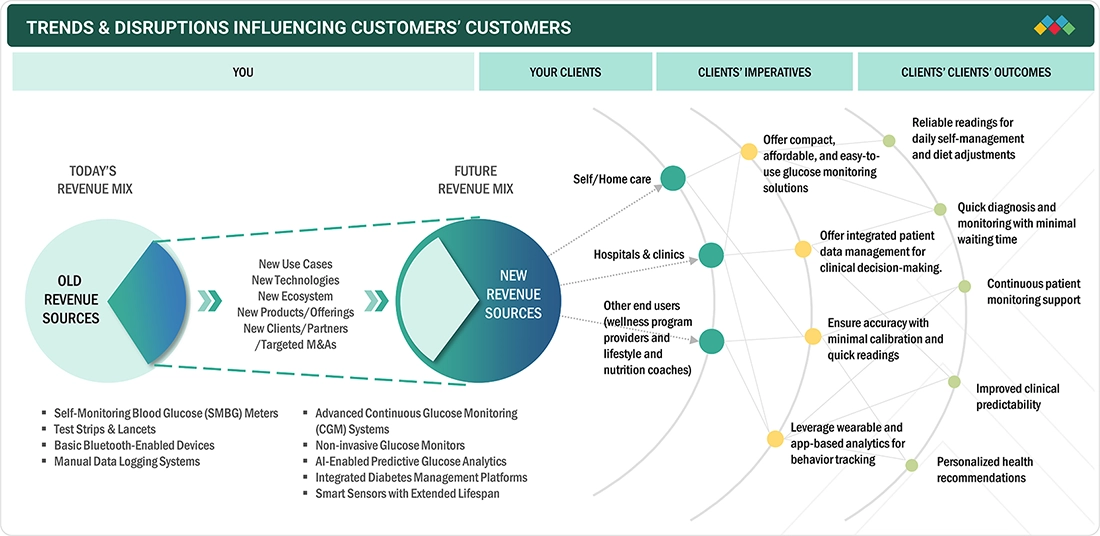
The Europe blood glucose monitor market is experiencing steady growth, supported by rising diabetes prevalence due to aging populations and lifestyle changes. Increasing adoption of advanced continuous and non-invasive monitoring technologies, combined with innovations in connectivity, mobile health integration, and smart analytics, is expanding their use in clinical and home-care settings.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The Europe blood glucose monitor market is experiencing a shift from traditional glucose meters and strip-based testing toward advanced, technology-driven solutions. Emerging trends include the adoption of CGM systems, non-invasive and minimally invasive sensors, smart connected devices, and AI-powered analytics. These innovations are creating high-growth opportunities, driven by the demand for real-time monitoring, improved accuracy, and enhanced diabetes management. End users such as hospitals, clinics, and home-care patients are increasingly adopting these disruptive technologies, reshaping the future of diabetes care.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing prevalence of diabetes

-
Growing elderly population and increasing life expectancy
Level
-
Short sensor lifespan and frequent replacements
Level
-
Integration with digital health platforms and smart devices
-
Expansion of coverage and reimbursement policies for CGMs
Level
-
Regulatory compliance challenges for non-invasive glucose monitoring technologies
-
Data overload and alarm fatigue
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Increasing prevalence of diabetes
The number of people suffering from diabetes in Europe is steadily increasing, and this is a major factor contributing to the demand for blood glucose monitoring devices. According to the International Diabetes Federation (IDF), about 65.6 million people, or 9.8% of the population of Europe, were suffering from diabetes in 2024, and this is expected to increase by 10% by 2050. The high and increasing prevalence of Type 1 and Type 2 diabetes, including a notable proportion of younger patients and the rapidly growing geriatric population, reinforces the need for accurate, convenient, and continuous glucose monitoring solutions. With diabetes representing a major public health concern across the region, healthcare systems face sustained pressure to support effective long-term disease management. This escalating prevalence and the associated healthcare burden continue to drive the demand for reliable, user-friendly, and technologically advanced blood glucose monitoring devices. This enables early detection, continuous monitoring, and improved patient outcomes across clinical and home-care settings.
Restraint: Short sensor lifespan and frequent replacements
The short lifespan of blood glucose monitoring sensors and the need for frequent replacements have been identified as a major limitation of the Europe blood glucose monitor market. Most continuous glucose monitoring (CGM) sensors are only meant to be worn for a short time, such as days or weeks, which leads to increased costs for the patient and the payer over time. Despite strong insurance coverage in the region, due to repeated sensor replacements, the out-of-pocket expenses of users of these monitoring devices constitute an issue for concern, particularly for uninsured or underinsured populations. Besides, frequent sensor changes can cause inconvenience and challenge adherence to the treatment plan for users, particularly for elderly patients and those with long-term diabetes. In addition, supply continuity, prescription renewals, and reimbursement limitations related to the frequency of replacements can hinder the consistent use of the device. These factors have a limiting influence on the wide, ranging market penetration and hamper the overall growth potential of blood glucose monitoring systems in Europe.
Opportunity: Integration with digital health platforms and smart devices
The integration of blood glucose monitors with digital health platforms and smart devices is emerging as a transformative opportunity in diabetes management. Advancements in Bluetooth and cloud connectivity allow real-time glucose data to be synchronized with smartphones, wearables, and electronic health records, enabling continuous monitoring and data-driven decision-making. Patients benefit from personalized alerts, automated insulin dose recommendations, and trend analytics that improve self-management and clinical outcomes. For healthcare providers, integrated platforms facilitate remote monitoring, teleconsultations, and population-level analytics, enhancing care coordination and reducing hospital visits. As governments and insurers increasingly support digital health adoption, this trend is expected to accelerate, positioning connected glucose monitoring as a cornerstone of next-generation diabetes care.
Challenge: Regulatory compliance challenges for non-invasive glucose monitoring technologies
Non-invasive glucose monitoring technologies face significant regulatory challenges as authorities such as the EU MDR require rigorous proof of safety, accuracy, and consistency across diverse populations and conditions. Unlike conventional blood-based systems, these devices rely on optical or electromagnetic sensing, which can be affected by skin tone, hydration, and environmental factors, complicating validation. The absence of standardized testing protocols increases uncertainty, leading to lengthy approval timelines and costly clinical studies. Consequently, despite strong innovation potential, regulatory and reimbursement barriers continue to delay the large-scale commercialization of non-invasive glucose monitoring solutions.
EUROPE BLOOD GLUCOSE MONITOR MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
FreeStyle Libre 3 continuous glucose monitoring (CGM) system designed for real-time glucose tracking without routine fingersticks; transmits minute-by-minute readings to a connected smartphone app | Eliminates fingerstick calibration | Enhances patient comfort | Enables proactive glucose management | Supports better glycemic control through trend-based insights |
 |
Dexcom G7 all-in-one CGM sensor providing real-time glucose readings, customizable alerts, and integration with smartphones and insulin pumps for seamless diabetes management | Offers superior accuracy and fast warm-up | Provides predictive alerts to prevent hypo- and hyperglycemia | Improves adherence through connectivity and data visualization |
 |
Guardian 3 Sensor used with Medtronic insulin pumps to deliver continuous glucose data every five minutes, supporting automated insulin delivery and trend-based therapy adjustments | Enables hybrid closed-loop insulin management | Improves time-in-range | Reduces glycemic variability | Enhances safety for insulin-dependent users |
 |
Omnitest self-monitoring blood glucose (SMBG) device designed for capillary blood testing, offering reliable and quick results for daily home or point-of-care glucose monitoring | Provides affordable, accurate, and easy-to-use glucose testing | Supports frequent monitoring | Improves patient engagement in self-managed care |
 |
Accu-Chek range of SMBG systems designed for fast and dependable glucose measurement with connectivity to digital platforms for progress tracking and data sharing | Ensures consistent accuracy | Simplifies diabetes management | Enables remote data access | Strengthens patient–clinician communication for improved outcomes |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe blood glucose monitor market operates within a complex ecosystem comprising multiple stakeholders that collectively drive innovation, distribution, and adoption. Device manufacturers develop accurate and reliable blood glucose monitoring systems, while distributors ensure these products reach hospitals, clinics, pharmacies, and retail channels. Healthcare providers, including hospitals and specialized diabetes care centers, integrate these devices into patient management, supported by doctors, nurses, and other professionals who guide usage and monitor outcomes. Patients and caregivers form the end user base, relying on these devices for daily diabetes management. Health advocacy organizations promote awareness, best practices, and policy support, while government and regulatory bodies ensure safety, efficacy, and compliance, shaping market access and product standards. This interconnected network defines the operational and innovation landscape of the global blood glucose monitor market.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe Blood Glucose Monitor Market, By Product
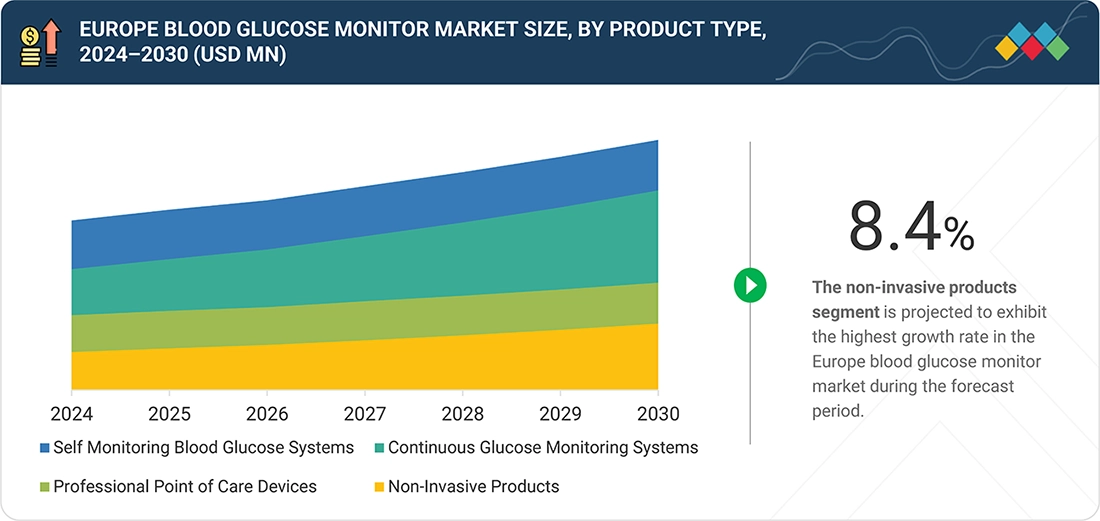
Based on product, the Europe blood glucose monitor market is segmented into self-monitoring blood glucose (SMBG) systems, continuous glucose monitoring (CGM) systems, professional point-of-care devices, and non-invasive products. Among these, CGM systems are expected to register the fastest growth during the forecast period, driven by rapid technological advancements and the increasing demand for continuous, real-time glucose monitoring. CGM devices reduce dependence on frequent finger-prick testing and support improved glycemic control through continuous trend analysis and early detection of glucose fluctuations. Their integration with digital health platforms, insulin delivery systems, and smartphone applications further enhances patient convenience and treatment personalization. In addition, the availability of CE-marked next-generation sensors and expanding reimbursement support across Europe are accelerating adoption. Notably, in June 2024, France became the first country in Europe to offer full national reimbursement for Dexcom CGM technology for patients with Type 2 diabetes treated with basal insulin injections, reinforcing the role of policy support in driving CGM uptake across the region.
Europe Blood Glucose Monitor Market, By Application
By application, the Europe blood glucose monitor market is segmented into diabetes management, health and wellness monitoring, and other applications, with the diabetes management segment expected to register the highest growth during the forecast period. The increasing prevalence of Type 1 and Type 2 diabetes, coupled with Europe’s aging population, has intensified the need for continuous and reliable glucose monitoring within structured care pathways. CGM systems are gaining traction by enabling real-time glucose insights that support treatment optimization, insulin titration, and lifestyle management. Integration with national digital health infrastructures and electronic health records facilitates data sharing between patients and healthcare providers, improving clinical oversight and personalized care. Regular monitoring enables early identification of glycemic variability, supporting better long-term disease control and reducing the risk of complications. In addition, public healthcare funding, disease management programs, and expanding reimbursement for glucose monitoring technologies across several European countries are improving access, while easy-to-use home monitoring solutions are strengthening patient engagement and adherence. These factors are expected to drive the diabetes management application segment across the region.
Europe Blood Glucose Monitor Market, By End User
Based on end user, the Europe blood glucose monitor market is segmented into self/home care, hospitals and clinics, and other end users. Among these, the self/home care segment accounts for the largest share, supported by the increasing shift toward home-based disease management within Europe’s publicly funded healthcare systems. Growing patient awareness of the importance of regular glucose monitoring, along with the rising number of individuals managing diabetes outside of hospital settings, has strengthened the demand for home-use devices. Broad availability of easy-to-use monitoring systems through community pharmacies and reimbursed supply channels has further supported adoption. In addition, advancements such as connected meters, continuous glucose monitoring (CGM) systems, and smartphone-based data tracking have improved usability and patient engagement. The expanding role of teleconsultations, remote follow-up, and preventive care models across Europe continues to reinforce the dominance of the self/home care segment in the blood glucose monitor market.
REGION
Germany to be fastest-growing country in Europe blood glucose monitor market during forecast period
The Germany blood glucose monitor market is expected to register the highest growth rate within Europe during the forecast period. Market expansion is driven by the large and steadily growing diabetes population, increasing awareness of the importance of regular glucose monitoring, and rising adoption of continuous and digitally connected glucose monitoring technologies. The aging population, along with a high prevalence of lifestyle-related conditions, is increasing the demand for accurate, convenient, and patient-friendly monitoring solutions across both home-care and outpatient settings. In addition, the strong healthcare expenditure base, comprehensive statutory health insurance coverage, and early uptake of digital health initiatives—telemedicine and remote patient monitoring—are supporting broader access to advanced glucose monitoring systems. Collectively, these factors position the country as a key growth market for blood glucose monitors in Europe.
EUROPE BLOOD GLUCOSE MONITOR MARKET: COMPANY EVALUATION MATRIX
In the Europe blood glucose monitor market matrix, Abbott Laboratories (Star) maintains a dominant position, driven by its strong regional footprint and comprehensive portfolio across continuous glucose monitoring (CGM) and self-monitoring blood glucose (SMBG) systems. Its leadership is supported by robust R&D capabilities, clinically proven accuracy, and advanced digital integration through the widely adopted FreeStyle Libre platform across homecare and clinical settings. i-SENS, Inc. (Emerging Leader) is gradually strengthening its presence in the region with accurate, cost-effective SMBG devices and early-stage expansion into CGM through its CareSens Air system. While Abbott continues to benefit from scale, innovation, and strong brand trust among providers and payers, i-SENS shows potential to progress toward the leaders’ quadrant by leveraging its manufacturing expertise, expanding distribution partnerships, and focus on connected, value-driven glucose monitoring solutions for price-sensitive segments in Europe.
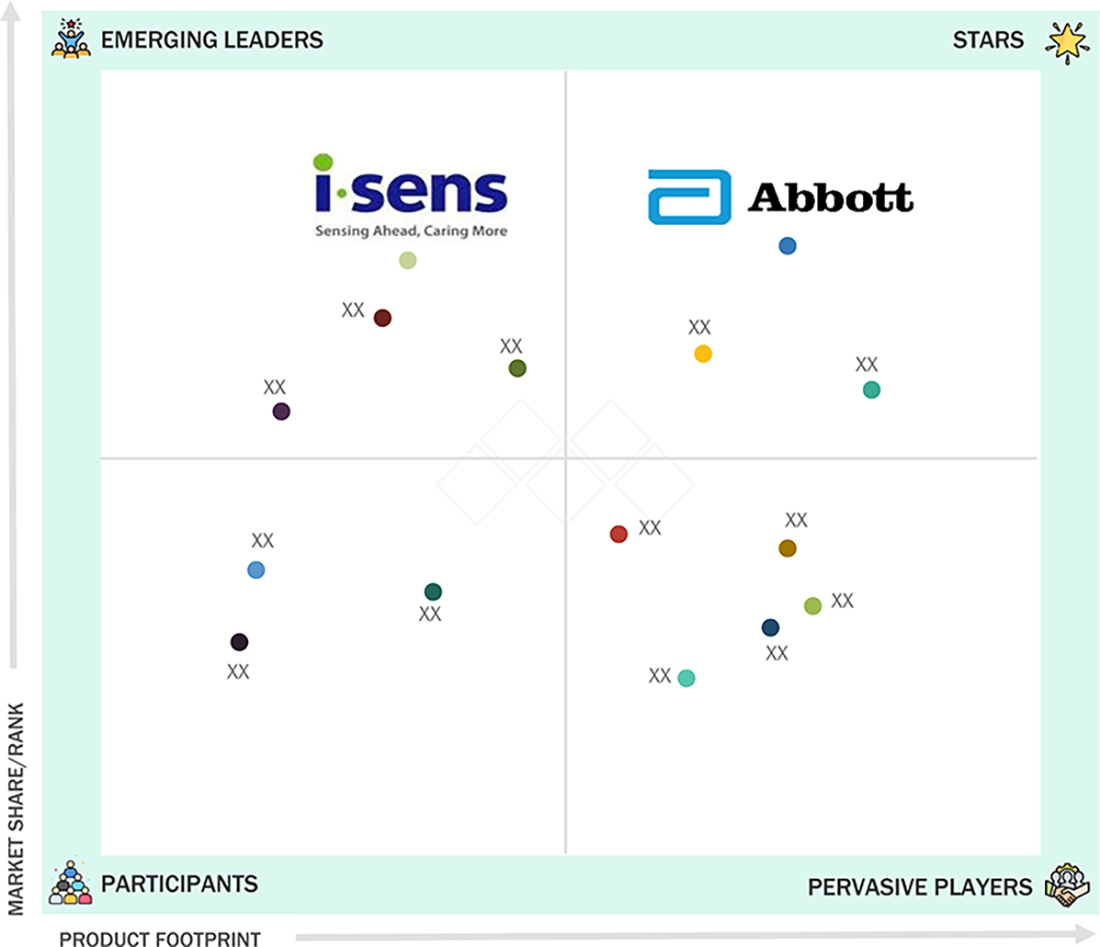
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Abbott Laboratories (US)
- Medtronic (Ireland)
- Dexcom (US)
- Ascensia Diabetes Care Holdings AG (Switzerland)
- Sinocare (China)
- F. Hoffmann-La Roche Ltd (Switzerland),
- B Braun SE (Germany)
- Nipro (Japan)
- Senseonics (US)
- i-Sens, Inc (South Korea)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 4.43 Billion |
| Market Forecast in 2030 (Value) | USD 6.85 Billion |
| Growth Rate | CAGR of 7.6% from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Million/Billion), Volume (Thousands Units) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered | By Product Type: Self Monitoring Blood Glucose Systems, Continuous Glucose Monitoring Systems, Professional Point of Care Devices, Non-invasive Products I Test Site: Fingertip, Upper Arm, Alternate Sites I By Application: Diabetes Management, Health & Wellness Monitoring, Other Applications I End User: Self/Home Care, Hospitals & Clinics, Other End Users |
| Countries Covered | France, Germany, the UK, Italy, Spain, and Rest of Europe |
WHAT IS IN IT FOR YOU: EUROPE BLOOD GLUCOSE MONITOR MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Volume Analysis | Market assessment by volume (units) for products such as self-monitoring blood glucose monitoring systems and continuous glucose monitoring systems |
|
| Company Information | Key players: Abbott Laboratories (US), DexCom, Inc. (US), Medtronic (Ireland), F. Hoffmann-La Roche Ltd. (Switzerland), B. Braun SE (Germany), and Ascensia Diabetes Care Holdings AG (Switzerland) Top 3-5 players market share analysis at APAC and the North American country level | Insights on revenue shifts toward emerging innovations |
| Disease Prevalence |
|
|
RECENT DEVELOPMENTS
- November 2024 : Abbott Laboratories (US) announced the opening of its state-of-the-art manufacturing facility in Kilkenny, Ireland. This site is a global manufacturing center of excellence for Abbott’s diabetes care business.
- March 2024 : i-Sense, Inc. (South Korea) announced receiving CE approval for CareSens Air, its continuous glucose monitoring (CGM) device. The CareSens Air CGM system offers a non-invasive solution for diabetes management, allowing users to monitor their glucose levels in real-time without the need for finger-prick blood samples.
- March 2022 : DexCom, Inc. (US) announced that it has received the CE Mark (Conformité Européenne) for the DexCom G7 Continuous Glucose Monitoring (CGM) System. This system is applicable to individuals with diabetes in Europe aged two years and older, including pregnant women.
Table of Contents

Methodology
The study comprised four key activities to estimate the current size of the europe blood glucose monitor market. First, extensive secondary research was conducted to gather information on the market, including related and parent markets. The next step involved validating these findings, assumptions, and market size estimates through primary research with industry experts across the value chain. Top-down and bottom-up approaches were used to arrive at a comprehensive estimate of the overall market size. Finally, market breakdown and data triangulation techniques were utilized to determine the sizes of segments and subsegments within the market.
Secondary Research
The secondary research process involved extensive use of various secondary sources, including directories, databases like Bloomberg Business, Factiva, and D&B Hoovers, as well as white papers, annual reports, company house documents, investor presentations, and SEC filings. This research was conducted to gather information valuable for a comprehensive, technical, market-oriented, and commercial study of the europe blood glucose monitor market. It also helped obtain critical insights about key players in the industry, market classification, and segmentation based on current industry trends, down to the finest details. Additionally, a database of leading industry players was created through this secondary research.
Primary Research
In the primary research process, interviews were conducted with various sources from the supply and demand sides to gather qualitative and quantitative information for this report. On the supply side, industry experts were interviewed, including CEOs, vice presidents, marketing & sales directors, technology & innovation directors, and other key executives from prominent companies and organizations involved in the europe blood glucose monitor market. For the demand side, interviews were conducted with industry experts, purchase and sales managers, doctors, and personnel from research organizations. This primary research was essential to validate market segmentation, identify key players in the industry, and gather insights on important industry trends and market dynamics.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The research methodology used to estimate the size of the europe blood glucose monitor market includes the following details. The market sizing was undertaken from the global side.
Country-level Analysis: The size of the europe blood glucose monitor market was obtained from the annual presentations of leading players and secondary data available in the public domain. The share of products in the overall europe blood glucose monitor market was obtained from secondary data and validated by primary participants to arrive at the total europe blood glucose monitor market. Primary participants further validated the numbers.
Geographic Market Assessment (By Region and Country): The geographic assessment was done using the following approaches:
Approach 1: Geographic revenue contributions/splits of leading players in the market (wherever available) and respective growth trends
Approach 2: Geographic adoption trends for individual product segments by end users and growth prospects for each of the segments (assumptions and indicative estimates validated from primary interviews)
At each point, the assumptions and approaches were validated by industry experts who were contacted during primary research. Considering the limitations of data available from secondary research, revenue estimates for individual companies (for the overall europe blood glucose monitor market and geographic market assessment) were ascertained based on a detailed analysis of their respective product offerings, geographic reach/strength (direct or through distributors or suppliers), and the shares of the leading players in a particular region or country.
Europe blood Glucose Monitor Market Size: Bottom-up Approach and Top-down Approach
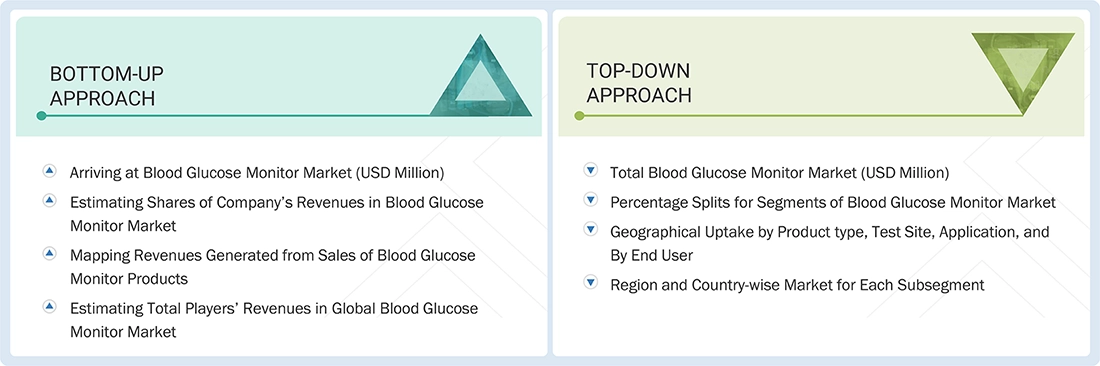
Data Triangulation
After arriving at the overall size from the market size estimation process explained above, the total market was split into several segments and subsegments. The data triangulation and market breakdown procedures explained below were implemented, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics for various market segments and subsegments. The data was triangulated by studying various factors and trends from the demand and supply sides. Along with this, the market size was validated using both the top-down and bottom-up approaches.
Market Definition
Europe blood Glucose monitors are medical devices used to measure and monitor europe blood Glucose (sugar) levels in individuals, primarily for the diagnosis and management of diabetes mellitus. These monitors help patients and healthcare professionals assess glycemic control, adjust treatment plans, and prevent diabetes-related complications.
Stakeholders
- Europe blood Glucose Monitor Manufacturers
- Contract Manufacturers
- Suppliers and Distributors of Europe Blood Glucose Monitors
- Senior Management
- Finance Department
- Healthcare Services Providers (Hospitals and Public & Private Clinics)
- Academic & Research Institutes
- E-commerce and Digital Platforms
- Retail Pharmacies and Supermarkets
- Trade Associations and Industry Bodies
- Regulatory Bodies and Government Agencies
- Business Research and Consulting Service Providers
- Market Research and Consulting Firms
- Venture Capitalists and Investors
Report Objectives
- To define, describe, segment, analyze, and forecast the North America Europe Blood Glucose monitor market by product type, application, test site, end user, and region.
- To provide detailed information about the factors influencing market growth (drivers, restraints, opportunities, and challenges)
- To analyze micromarkets concerning individual growth trends, prospects, and contributions to the overall market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To forecast the size of the europe blood glucose monitor market in North America, Europe, the Asia Pacific, Latin America, the Middle East & Africa, and GCC countries
- To profile the key players in the europe blood glucose monitor market and comprehensively analyze their core competencies
- To track and analyze competitive developments such as agreements, collaborations, and partnerships; acquisitions; and product approvals & launches in the europe blood glucose monitor market.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Blood Glucose Monitor Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in Europe Blood Glucose Monitor Market