
Liquid Biopsy Market Size, Growth, Share & Trends Analysis
Liquid Biopsy Market by Product & Service (Kits, Instruments), Circulating Biomarker (ctDNA, cfDNA, CTC), Technology (NGS, PCR), Application (Lung, Breast, Prostate Cancer), Sample Type (Blood, Urine, CSF), End User (Hospitals) - Global Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
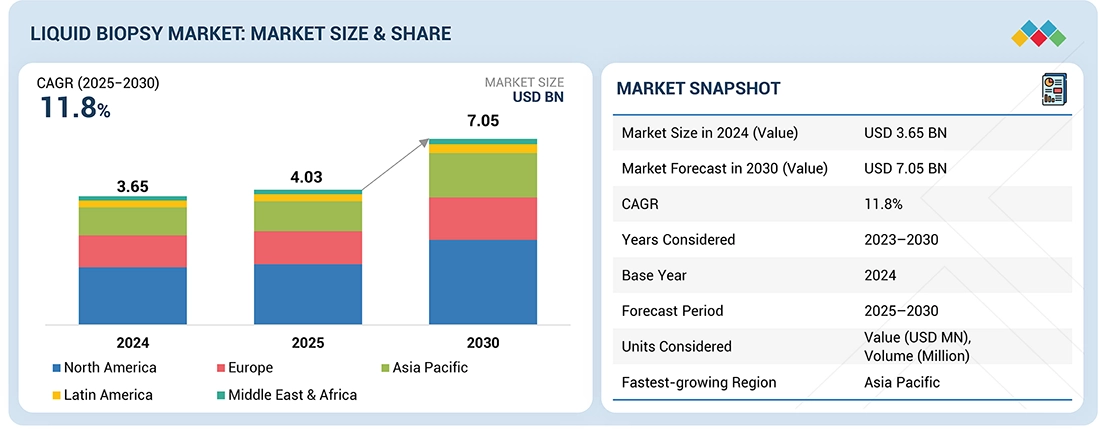
The liquid biopsy market is estimated at USD 4.03 billion in 2025 and is projected to reach USD 7.05 billion by 2030, growing at a CAGR of 11.8% during the forecast period. The liquid biopsy market is witnessing strong growth due to the rising global burden of cancer and increasing awareness of the importance of early detection, supported by initiatives from leading health organizations. Liquid biopsy provides several advantages over traditional tissue biopsy procedures, including being minimally invasive, offering faster results, and allowing real-time monitoring of disease progression and treatment response. These benefits make liquid biopsy an increasingly preferred option for both clinicians and patients. Additionally, advancements in molecular technologies and the expanding adoption of personalized medicine are further enhancing the appeal of liquid biopsy as a reliable and efficient diagnostic tool across various cancer types.
KEY TAKEAWAYS
-
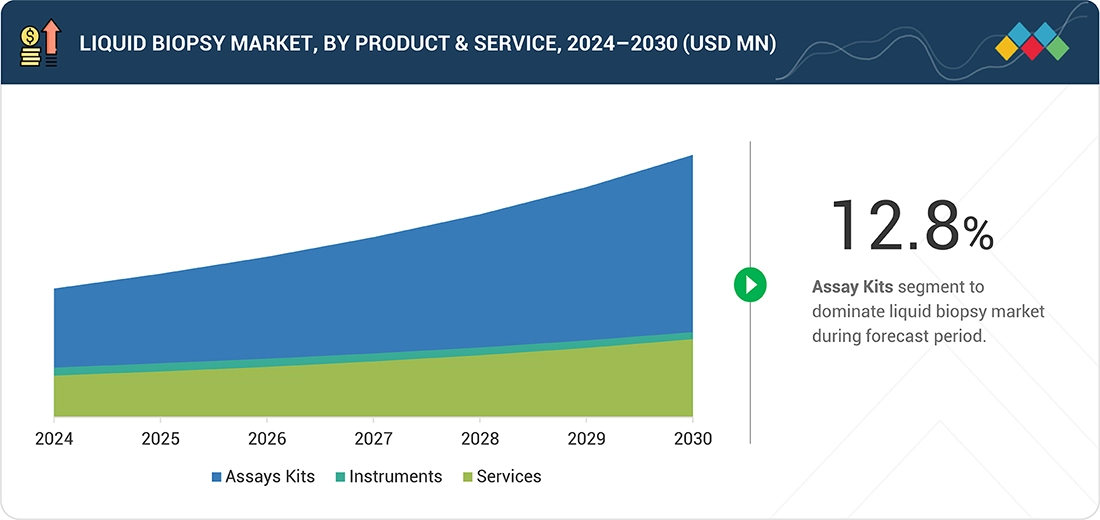
BY PRODUCT & SERVICEBased on product and service, the liquid biopsy market is segmented into assay kits, instruments, and services. Among these, assay kits continue to see the widest use, as they are routinely required and frequently replenished by end users. Their role in enabling reliable testing, coupled with the growing adoption of liquid biopsy across oncology and other clinical applications, is reinforcing their demand. Furthermore, the need for highly specific and sensitive assays to support early cancer detection, treatment monitoring, and companion diagnostics is expected to strengthen the growth of this segment in the years ahead.
-
BY CIRCULATING BIOMARKER TYPECategorized by circulating biomarker type, the liquid biopsy market is segmented into circulating tumour cells, circulating tumour DNA (ctDNA), cell-free DNA, extracellular vesicles, and other biomarkers. Among these, ctDNA is gaining rapid traction owing to its growing role in cancer detection, treatment monitoring, and identification of minimal residual disease. By providing detailed genetic insights from a simple blood draw, ctDNA serves as a less invasive and more practical alternative to tissue biopsies. Its ability to track tumour evolution over time also makes it highly valuable in guiding therapy choices. With increasing clinical adoption and expanding regulatory support, ctDNA-based applications are expected to play an increasingly central role in cancer care.
-
BY TECHNOLOGYThe liquid biopsy market covers technologies such as multi-gene parallel analysis using next-generation sequencing (NGS) and single-gene analysis with PCR & microarrays. NGS-based multi-gene analysis is seeing rapid uptake because it enables the simultaneous detection of multiple genetic alterations, offering detailed molecular profiling with high accuracy. Its high-throughput nature and adaptability make it well suited for identifying complex genomic changes, which is essential for applications like therapy selection and monitoring treatment response. With its growing role in personalized medicine and increasing incorporation into routine diagnostics, NGS continues to strengthen its position in cancer care.
-
BY APPLICATIONThe liquid biopsy market is divided into cancer and non-cancer applications, with cancer applications driving the majority of adoption. The rising global incidence of cancer and the growing focus on oncology research have positioned liquid biopsy as a vital tool in cancer management. Its ability to track tumor progression in real time, detect minimal residual disease, and address the limitations of traditional tissue biopsies makes it especially valuable. The increasing integration with companion diagnostics and continuous technological advancements are further expanding its role in guiding treatment decisions and improving patient outcomes.
-
BY CLINICAL APPLICATIONBased on clinical application, the liquid biopsy market is segmented into early cancer screening, therapy selection, treatment monitoring, and recurrence monitoring. Therapy selection represents the largest application area, supported by liquid biopsy’s ability to provide detailed molecular insights that guide personalized treatment decisions. By detecting actionable genetic alterations, these tests help match patients with targeted therapies, enhancing treatment effectiveness and outcomes. The rising adoption of precision medicine and the demand for minimally invasive methods to determine therapy suitability are further driving growth in this segment. In addition, ongoing advancements and the integration of liquid biopsy with companion diagnostics continue to reinforce its importance in oncology care.
-
BY SAMPLE TYPELiquid biopsy samples are primarily classified into blood and other types, with blood being the most widely used due to its ease of collection, minimal invasiveness, and ability to provide comprehensive molecular insights from biomarkers such as ctDNA, cfDNA, and CTCs. A simple blood draw offers critical information for cancer detection, therapy selection, treatment monitoring, and recurrence assessment, making it practical for routine clinical use. Compared with traditional tissue biopsies, blood-based sampling is safer, faster, and more acceptable to patients, driving its broad adoption in both diagnostic and research settings. Ongoing technological advancements and increasing regulatory support are expected to keep blood as the preferred sample type in liquid biopsy applications.
-
BY END USERThe liquid biopsy market caters to a range of end users, including reference laboratories, hospitals and physician laboratories, academic & research centers, and other healthcare settings. Reference laboratories are the primary users, owing to their high testing volumes, advanced infrastructure, and ability to perform complex molecular analyses. These laboratories play a crucial role in supporting oncology diagnostics, companion diagnostics, and clinical research studies. Hospitals, physician laboratories, and academic institutes also contribute to adoption by offering decentralized testing and specialized services. The growing demand for minimally invasive cancer diagnostics and the increasing use of liquid biopsy in clinical and research applications are driving market uptake across all end-user segments.
The liquid biopsy market is set to experience substantial growth in the coming years, driven by the increasing global burden of cancer, rising awareness of early detection, and ongoing advancements in molecular diagnostic technologies. Minimally invasive liquid biopsy techniques, including circulating tumor DNA (ctDNA) and circulating tumor cell (CTC) analyses, are being increasingly utilized to provide rapid and accurate insights into disease status, enabling timely clinical decisions and personalized treatment planning. Expanding adoption of liquid biopsy is supported by its advantages over traditional tissue biopsies, such as reduced risk, faster results, and the ability to monitor disease progression in real time. Moreover, growing emphasis on precision medicine and the integration of companion diagnostics are further accelerating uptake, while continuous technological innovation and expanding accessibility across hospitals, clinics, and specialized testing centers are enhancing the efficiency, reliability, and clinical impact of liquid biopsy solutions.
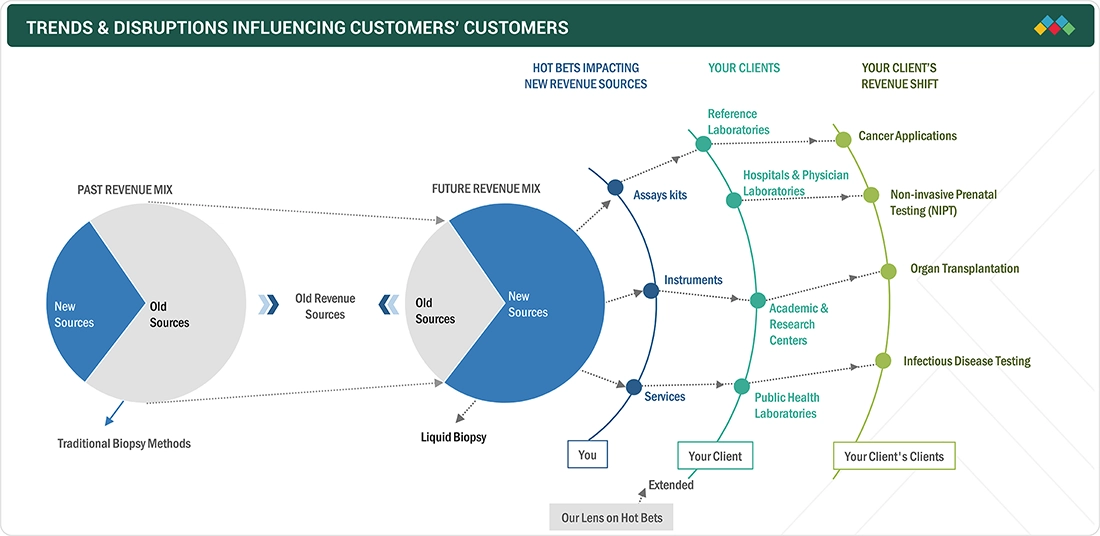
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The liquid biopsy market is witnessing rapid growth, driven by the increasing prevalence of cancer, a stronger focus on early detection, and ongoing advancements in molecular diagnostic technologies. Liquid biopsy solutions are primarily utilized by reference laboratories, hospitals, physician laboratories, and academic and research institutes. The adoption of minimally invasive testing, combined with the integration of companion diagnostics and innovations in multi-gene and targeted assays, is transforming clinical workflows and improving patient management. This rising demand is boosting the use of assay kits, instruments, and associated services, promoting wider implementation of liquid biopsy across healthcare and research settings.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing burden of cancer

-
Cancer awareness initiatives undertaken by global health organizations
Level
-
Lower sensitivity of certain liquid biopsy procedures
Level
-
Growing significance of companion diagnostics
-
Growth potential of emerging economies
Level
-
Unfavourable reimbursement scenario
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Increasing burden of cancer
The rising global incidence and prevalence of cancer is a key driver of the liquid biopsy market. As cancer cases continue to increase, there is a growing need for diagnostic solutions that enable early detection, treatment guidance, and monitoring of disease progression. Liquid biopsy provides a minimally invasive and repeatable alternative to traditional tissue biopsies, allowing detection of circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and other biomarkers. Its suitability for repeated monitoring and early diagnosis makes it particularly valuable in oncology care. With cancer becoming more widespread, healthcare systems are increasingly adopting technologies that facilitate timely and accurate clinical decision-making, further boosting the demand for liquid biopsy products and services.
Restraint: Lower sensitivity of certain liquid biopsy procedures
Lower sensitivity and specificity in certain liquid biopsy procedures remain a notable limitation, especially when detecting circulating tumor DNA (ctDNA). In some cases, the concentration of ctDNA in plasma may be extremely low, making it difficult to detect and potentially leading to false-negative results. Such limitations reduce the diagnostic and clinical reliability of liquid biopsy, as the test may fail to identify existing cancer-related biomarkers. Statistical and sampling challenges further exacerbate this issue, particularly in early-stage cancers or minimal residual disease monitoring. Consequently, delayed detection of tumor recurrence or progression can occur, potentially impacting treatment decisions and patient outcomes.
Opportunity: Growing significance of companion diagnostics
The growing importance of companion diagnostics offers significant opportunities for the liquid biopsy market. As precision medicine continues to expand, companion diagnostics are increasingly used to identify patients who are most likely to benefit from specific therapies. Liquid biopsy, with its ability to non-invasively detect actionable biomarkers, is well-suited to serve this role. This has fostered collaboration between pharmaceutical and diagnostic companies, enabling the co-development of targeted therapies and accelerating the adoption of liquid biopsy in personalized cancer care.
Challenge: Unfavourable reimbursement scenario
Uncertainty in the reimbursement landscape presents a significant challenge for the adoption of liquid biopsy. High-cost molecular tests, particularly newer technologies, often face complex and inconsistent coverage policies. Interpreting these tests requires specialized expertise, and the associated costs are frequently unclear to physicians, pathologists, and patients until after billing or reimbursement. While some payers may cover multi-gene panels, reimbursement is sometimes limited to single-gene analysis, restricting the test’s clinical utility. This lack of clarity can make diagnostics unaffordable for many patients, underscoring the need for healthcare providers and stakeholders to stay informed about evolving reimbursement policies to ensure access and affordability.
Liquid Biopsy Market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Provides liquid biopsy solutions for cancer detection, therapy selection, minimal residual disease monitoring, and recurrence tracking. | Non-invasive and highly sensitive testing, supports early detection and ongoing monitoring, enables personalized treatment decisions, scalable for clinical and research use, integrated with companion diagnostics. |
 |
Offers ctDNA-based liquid biopsy tests for comprehensive genomic profiling and therapy guidance in multiple cancer types. | Rapid and reliable tumor profiling, minimally invasive, supports precision medicine and treatment selection, allows real-time monitoring of treatment response and disease progression, widely adopted in clinical oncology. |
 |
Supplies liquid biopsy assays for cancer risk assessment, therapy selection, and treatment monitoring across diverse oncology indications. | High reproducibility and accuracy, integration with companion diagnostics, supports early detection and therapy optimization, improves patient management, enhances research and clinical trial capabilities. |
 |
Provides NGS-based liquid biopsy platforms for multi-gene profiling, biomarker discovery, and personalized cancer genomics research. | High-throughput and precise sequencing, supports multi-gene and targeted analysis, enables comprehensive tumor characterization, facilitates clinical decision-making, enhances development of personalized oncology therapies. |
 |
Offers liquid biopsy assays and integrated platforms for oncology applications, including therapy selection, monitoring, and recurrence detection. | Accurate and reproducible results, supports multiplex testing and multi-biomarker analysis, compatible with clinical workflows, non-invasive and minimally disruptive, enables timely treatment decisions and continuous patient monitoring. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The liquid biopsy market serves a variety of end users, including reference laboratories, hospitals and physician laboratories, academic and research centers, and other healthcare settings. Testing primarily focuses on the non-invasive detection of cancer biomarkers, therapy selection, treatment monitoring, and recurrence assessment. Adoption is being driven by advancements in multi-gene and targeted assays, NGS-based platforms, and integrated testing workflows, which improve accuracy, turnaround time, and clinical utility. Growing emphasis on precision medicine, increasing awareness of early cancer detection, and the integration of liquid biopsy with companion diagnostics are further supporting the uptake of these solutions across diverse end-user segments.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Liquid Biopsy Market, By Product & Service
Based on product and service, the liquid biopsy market is segmented into assay kits, instruments, and services. Among these, assay kits continue to see the widest use, as they are routinely required and frequently replenished by end users. Their role in enabling reliable testing, coupled with the growing adoption of liquid biopsy across oncology and other clinical applications, is reinforcing their demand. Furthermore, the need for highly specific and sensitive assays to support early cancer detection, treatment monitoring, and companion diagnostics is expected to strengthen the growth of this segment in the years ahead.
Liquid Biopsy Market, By Circulating Biomarker Type
Categorized by circulating biomarker type, the liquid biopsy market is segmented into circulating tumour cells, circulating tumour DNA (ctDNA), cell-free DNA, extracellular vesicles, and other biomarkers. Among these, ctDNA is gaining rapid traction owing to its growing role in cancer detection, treatment monitoring, and identification of minimal residual disease. By providing detailed genetic insights from a simple blood draw, ctDNA serves as a less invasive and more practical alternative to tissue biopsies. Its ability to track tumour evolution over time also makes it highly valuable in guiding therapy choices. With increasing clinical adoption and expanding regulatory support, ctDNA-based applications are expected to play an increasingly central role in cancer care.
Liquid Biopsy Market, By Technology
The liquid biopsy market covers technologies such as multi-gene parallel analysis using next-generation sequencing (NGS) and single-gene analysis with PCR & microarrays. NGS-based multi-gene analysis is seeing rapid uptake because it enables the simultaneous detection of multiple genetic alterations, offering detailed molecular profiling with high accuracy. Its high-throughput nature and adaptability make it well suited for identifying complex genomic changes, which is essential for applications like therapy selection and monitoring treatment response. With its growing role in personalized medicine and increasing incorporation into routine diagnostics, NGS continues to strengthen its position in cancer care.
Liquid Biopsy Market, By Application
The liquid biopsy market is divided into cancer and non-cancer applications, with cancer applications driving the majority of adoption. The rising global incidence of cancer and the growing focus on oncology research have positioned liquid biopsy as a vital tool in cancer management. Its ability to track tumor progression in real time, detect minimal residual disease, and address the limitations of traditional tissue biopsies makes it especially valuable. The increasing integration with companion diagnostics and continuous technological advancements are further expanding its role in guiding treatment decisions and improving patient outcomes.
Liquid Biopsy Market, By Clinical Application
Based on clinical application, the liquid biopsy market is segmented into early cancer screening, therapy selection, treatment monitoring, and recurrence monitoring. Therapy selection represents the largest application area, supported by liquid biopsy’s ability to provide detailed molecular insights that guide personalized treatment decisions. By detecting actionable genetic alterations, these tests help match patients with targeted therapies, enhancing treatment effectiveness and outcomes. The rising adoption of precision medicine and the demand for minimally invasive methods to determine therapy suitability are further driving growth in this segment. In addition, ongoing advancements and the integration of liquid biopsy with companion diagnostics continue to reinforce its importance in oncology care.
Liquid Biopsy Market, By Sample type
Liquid biopsy samples are primarily classified into blood and other types, with blood being the most widely used due to its ease of collection, minimal invasiveness, and ability to provide comprehensive molecular insights from biomarkers such as ctDNA, cfDNA, and CTCs. A simple blood draw offers critical information for cancer detection, therapy selection, treatment monitoring, and recurrence assessment, making it practical for routine clinical use. Compared with traditional tissue biopsies, blood-based sampling is safer, faster, and more acceptable to patients, driving its broad adoption in both diagnostic and research settings. Ongoing technological advancements and increasing regulatory support are expected to keep blood as the preferred sample type in liquid biopsy applications.
Liquid Biopsy Market, By End User
The liquid biopsy market caters to a range of end users, including reference laboratories, hospitals and physician laboratories, academic & research centers, and other healthcare settings. Reference laboratories are the primary users, owing to their high testing volumes, advanced infrastructure, and ability to perform complex molecular analyses. These laboratories play a crucial role in supporting oncology diagnostics, companion diagnostics, and clinical research studies. Hospitals, physician laboratories, and academic institutes also contribute to adoption by offering decentralized testing and specialized services. The growing demand for minimally invasive cancer diagnostics and the increasing use of liquid biopsy in clinical and research applications are driving market uptake across all end-user segments.
REGION
North America accounted for the largest share of the global liquid biopsy market during the forecast period.
The liquid biopsy market in North America is growing due to advanced healthcare infrastructure, increasing awareness of early cancer detection, and the rapid adoption of precision oncology solutions. Strong research and development capabilities, significant investment in cancer diagnostics, and the presence of leading diagnostic companies further support the uptake of liquid biopsy technologies. These factors are driving adoption across hospitals, reference laboratories, physician laboratories, and academic research institutes, enabling non-invasive cancer detection, therapy selection, and ongoing monitoring.
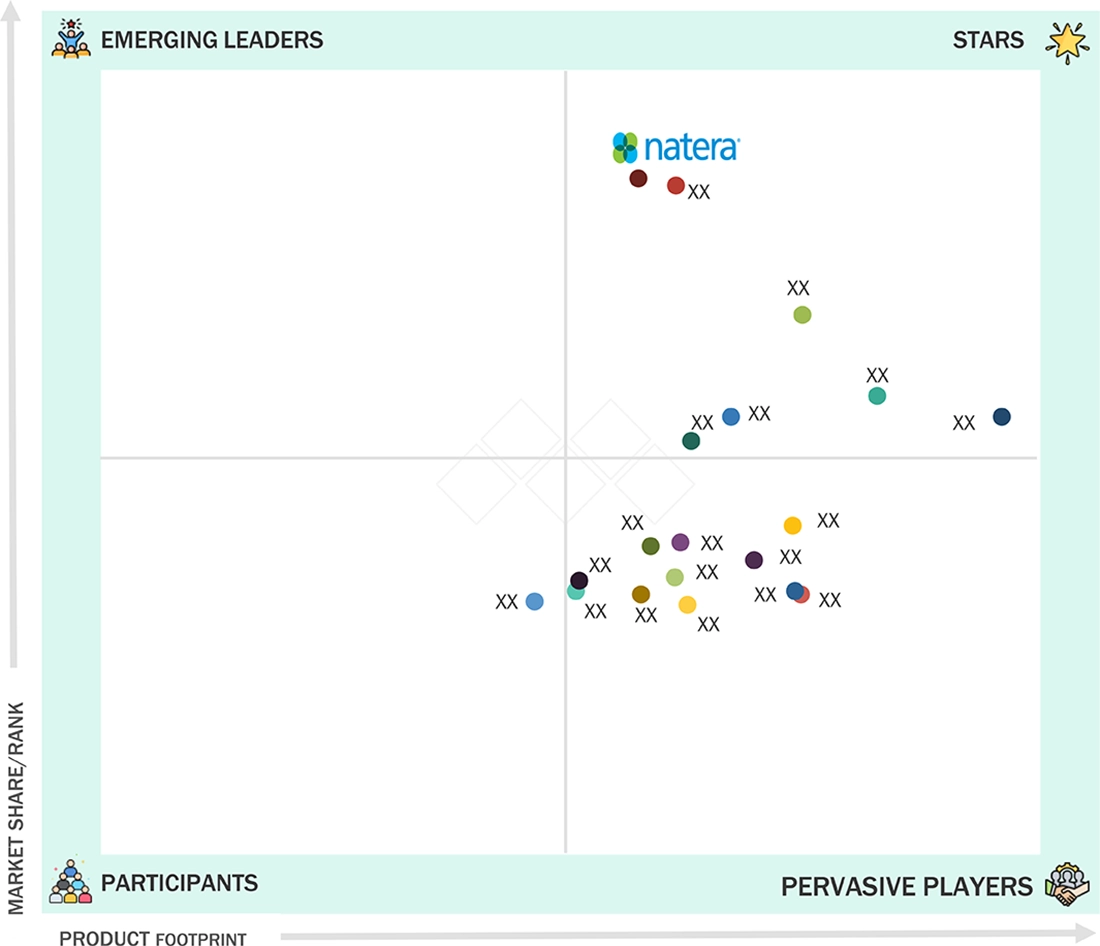
Liquid Biopsy Market: COMPANY EVALUATION MATRIX
Natera is a leading provider in the liquid biopsy market, specializing in non-invasive tests for cancer detection, therapy selection, and disease monitoring. The company serves hospitals, reference laboratories, physician labs, and research institutes, addressing a broad range of clinical and research needs. By partnering with healthcare providers and pharmaceutical companies, Natera enhances access to highly sensitive and accurate liquid biopsy solutions, supporting the integration of precision oncology into routine care in both developed and emerging markets.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size Value in 2024 | USD 3.65 Billion |
| Revenue Forecast in 2030 | USD 7.05 Billion |
| Growth Rate | CAGR of 11.8% from 2025-2030 |
| Actual data | 2023-2030 |
| Base year | 2024 |
| Forecast period | 2025-2030 |
| Units considered | Value (USD Million), Volume (Million) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered | By product & service (assay kits, instruments, and services), by circulating biomarker type (circulating tumor cells, circulating tumor DNA, cell-free DNA, extracellular vesicles and other circulating biomarkers), by technology (multi-gene parallel analysis using NGS and single-gene analysis using PCR & microarrays), by application (cancer applications [lung cancer, breast cancer, colorectal cancer, prostate cancer, melanoma, and other cancers] and non-cancer applications [non-invasive prenatal testing, organ transplantation, and infectious disease testing]), by clinical application (early cancer screening, therapy selection, treatment monitoring, and recurrence monitoring), by sample type (blood and other sample types, by end user (reference laboratories, hospitals and physician laboratories, academic & research centers, and other end users) |
| Regional Scope | North America, Europe, Asia Pacific, Latin America and the Middle East & Africa |
WHAT IS IN IT FOR YOU: Liquid Biopsy Market REPORT CONTENT GUIDE
RECENT DEVELOPMENTS
- April 2025 : Natera, Inc. (US), launched its ultra-sensitive Signatera Genome-MRD test in the US.
- February 2025 : Myriad Genetics, Inc. (US) collaborated with Gabbi (US), a telehealth solution for breast cancer risk assessment and specialist care services. The companies will work together to provide an integrated offering that combines Gabbi’s risk assessment program and access to breast specialists with Myriad’s MyRisk with RiskScore Hereditary Cancer Test.
- February 2024 : Myriad Genetics, Inc. (US) entered into a definitive agreement to acquire select assets from Intermountain Health. These included assets from its Intermountain Precision Genomics (IPG) laboratory business, including the Precise Tumor Test, the Precise Liquid Test, and IPG’s CLIA-certified laboratory.
- January 2024 : Natera, Inc. (US) acquired certain assets relating to non-invasive prenatal and carrier screening business from Invitae (US).
Table of Contents

Methodology
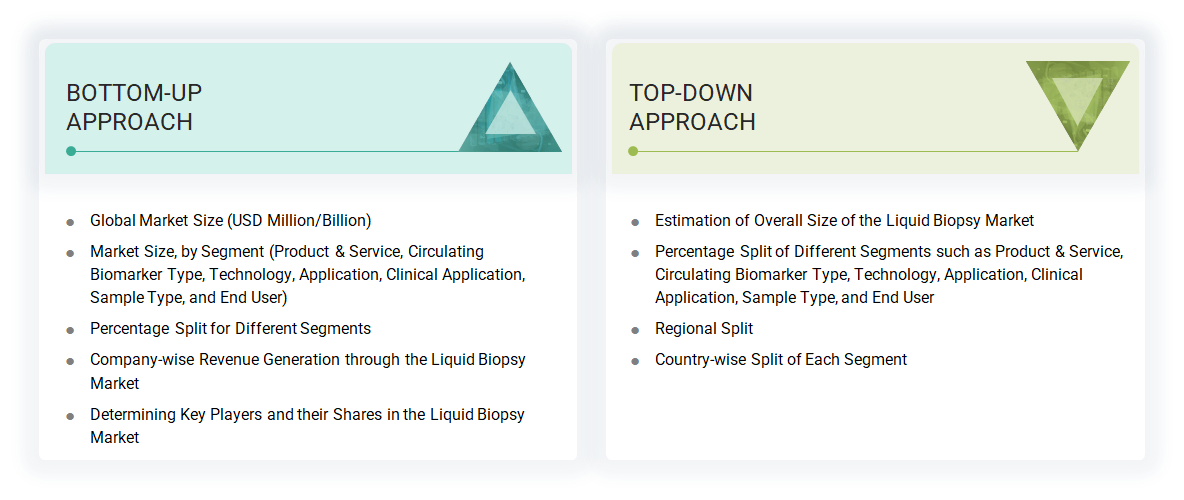
The study involved estimating activities to determine the current size of the liquid biopsy market. Exhaustive secondary research was done to collect information on the liquid biopsy industry. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain using primary research. Different approaches, such as top-down and bottom-up, were employed to estimate the total market size. After that, the market breakup and data triangulation procedures were used to estimate the market size of the segments and subsegments of the liquid biopsy market.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to identify and collect information for this study.
Primary Research
In the primary research process, various supply and demand sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources were mainly industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, service providers, technology developers, researchers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with primary respondents, including key industry participants, subject-matter experts, C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess prospects.
The following is a breakdown of the primary respondents:
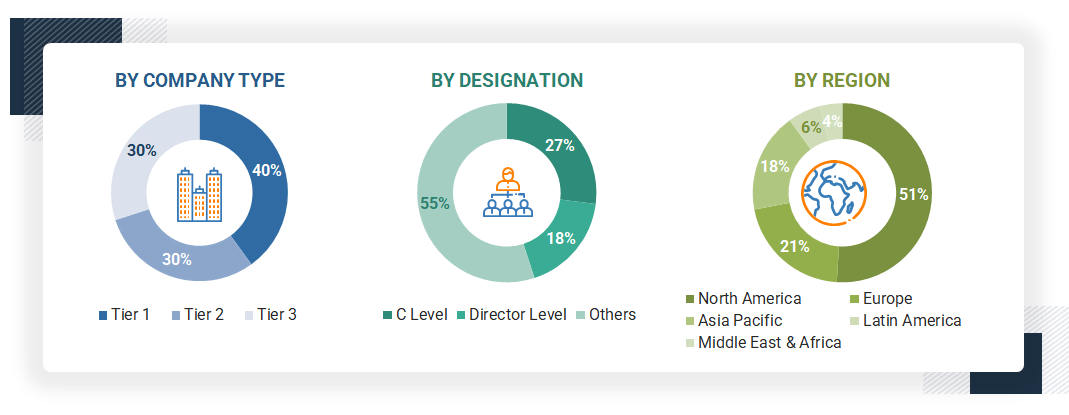
Note 1: Others include sales, marketing, and product managers.
Note 2: Companies are classified into tiers based on their total revenues. As of 2024, Tier 1 = >USD 100 million, Tier 2 = USD 10 million to USD 100 million, and Tier 3 = < USD 10 million.
To know about the assumptions considered for the study, download the pdf brochure
| COMPANY NAME | DESIGNATION |
|---|---|
| QIAGEN | Sales Manager |
| F. Hoffmann-La Roche Ltd. | Sales Executive |
Market Size Estimation
Liquid biopsy is a non-invasive method for diagnosing and monitoring diseases. Blood or other body fluids are collected to analyze cell-free DNA (cfDNA), circulating tumor DNA (ctDNA), or circulating tumor cells (CTCs).
Data Triangulation
After arriving at the overall market size by applying the abovementioned process, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand & supply sides.
Market Definition
Both top-down and bottom-up approaches were used to estimate and validate the liquid biopsy market's total size. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry have been identified through extensive secondary research
- Primary and secondary research have determined the revenues of leading players operating in the liquid biopsy market.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
The research methodology used to estimate the market size includes the following:
Stakeholders
- Manufacturers & Distributors of Liquid Biopsy Products
- Hospitals & Diagnostic Centers
- Reference Laboratories
- R&D Companies
- Research Laboratories and Academic Institutes
- Liquid Biopsy Service Providers
- Government Associations
- Venture Capitalists & Investors
Report Objectives
- To define, describe, segment, and forecast the liquid biopsy market, by product & service, circulating biomarker type, technology, application, clinical application, sample type, end user, and region
- To provide detailed information regarding the major factors influencing market growth (drivers, restraints, opportunities, and challenges)
- To analyze micro markets with respect to individual growth trends, prospects, and contributions to the overall market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To forecast the size of the market segments with respect to five regions, namely, North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
- To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze company developments such as acquisitions, collaborations, partnerships, agreements, and product launches & approvals in the liquid biopsy market
- To benchmark players within the liquid biopsy market using the Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business strategy, market share, and product offerings
Frequently Asked Questions (FAQ)
What are the recent trends affecting the liquid biopsy market?
The recent trends shaping the liquid biopsy market include the rising global cancer burden, the expansion of liquid biopsy applications beyond oncology, and the growing recognition of its advantages over traditional tissue biopsy methods.
What are the primary products categorized in the liquid biopsy market?
The liquid biopsy market is broadly segmented into assay kits, instruments, and services. The assay kits segment accounted for the largest share of the market in 2024. Assay kits, known for their cost-effectiveness and minimally invasive application, are vital in patient monitoring and cancer screening.
Who are the key players in the liquid biopsy market?
The key players in this market are Natera, Inc. (US), QIAGEN (Netherlands), Guardant Health (US), Illumina, Inc. (US), F. Hoffmann-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (US), Myriad Genetics, Inc. (US), Bio-Rad Laboratories, Inc. (US), Exact Sciences Corporation (US), and Sysmex Corporation (Japan).
What are the key applications of liquid biopsy?
The liquid biopsy market is segmented into cancer and non-cancer applications. In 2024, the cancer applications segment accounted for the largest share of the market. This dominance is attributed to the unique advantages liquid biopsies offer in oncology, including the ability to monitor tumor dynamics in real time, detect minimal residual disease, and support the use of companion diagnostics.
Which region is lucrative for the liquid biopsy market?
The Asia Pacific region is expected to grow at the highest CAGR during the forecast period.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Liquid Biopsy Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free CustomisationGrowth opportunities and latent adjacency in Liquid Biopsy Market













Elijah
Oct, 2022
Which of the end user segment is expected to hold the major share of the global Liquid Biopsy Market?.
Adam
Oct, 2022
What will be the global market value for Liquid Biopsy Market in 2029?.