
Asia Pacific Vaccines Market
Asia Pacific Vaccines Market by Technology (Conjugate, Recombinant, Inactivated, Live Attenuated, Viral Vector), Type (Monovalent, Multivalent), Disease (Pneumococcal, Flu, Hepatitis, MMR), Route of Administration (IM, SC, Oral) - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
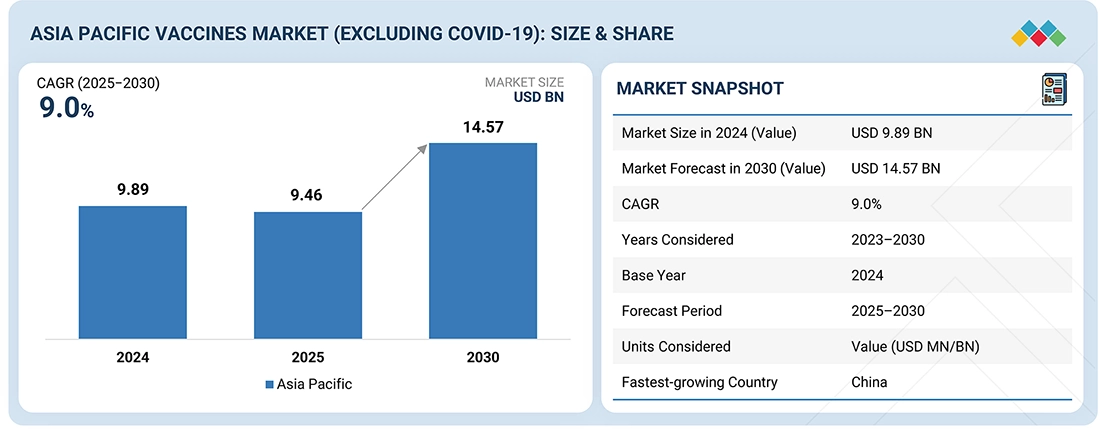
The Asia Pacific Vaccines market, valued at USD 9.46 billion in 2024, stood at USD 9.89 billion in 2025 and is projected to advance at a resilient CAGR of 9.0% from 2025 to 2030, culminating in a forecasted valuation of USD 14.57 billion by the end of the period. The market is expanding quickly, driven by a rise in vaccine development and commercialization, heightened infectious disease rates, and the extensive use of preventive vaccines. Other contributing factors are robust immunization programs, innovations in vaccine technology, and substantial government funding and investments in new vaccines for various diseases.
KEY TAKEAWAYS
-
BY COUNTRYChina is expected to grow at the highest CAGR of 10.8 % during the forecast period.
-
BY TYPEThe multivalent vaccines segment held 68.7% of the total market in 2024.
-
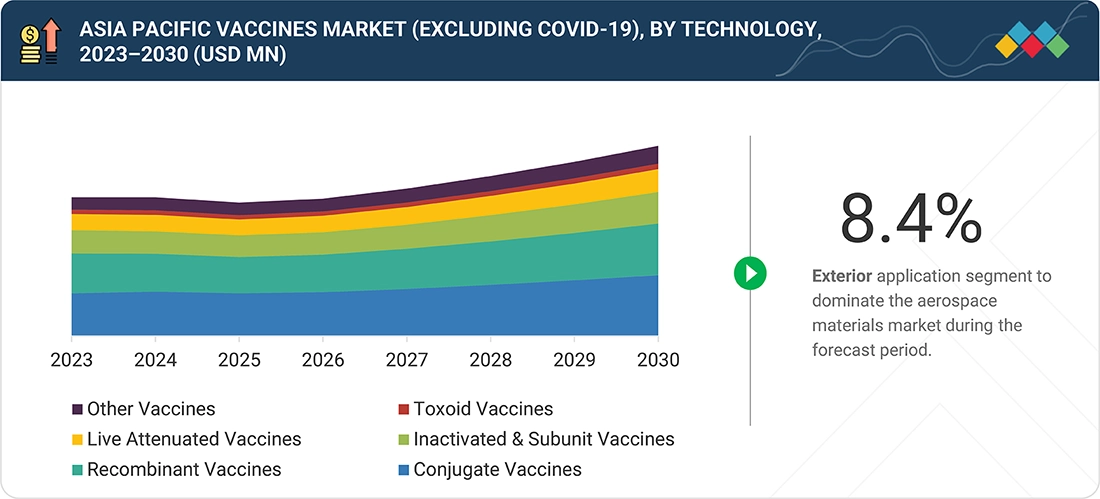
BY TECHNOLOGYThe inactivated & subunit vaccines segment is expected to record a CAGR of 9.6% during the forecast period.
-
BY DISEASEPneumococcal diseases dominate the Asia Pacific vaccines market.
-
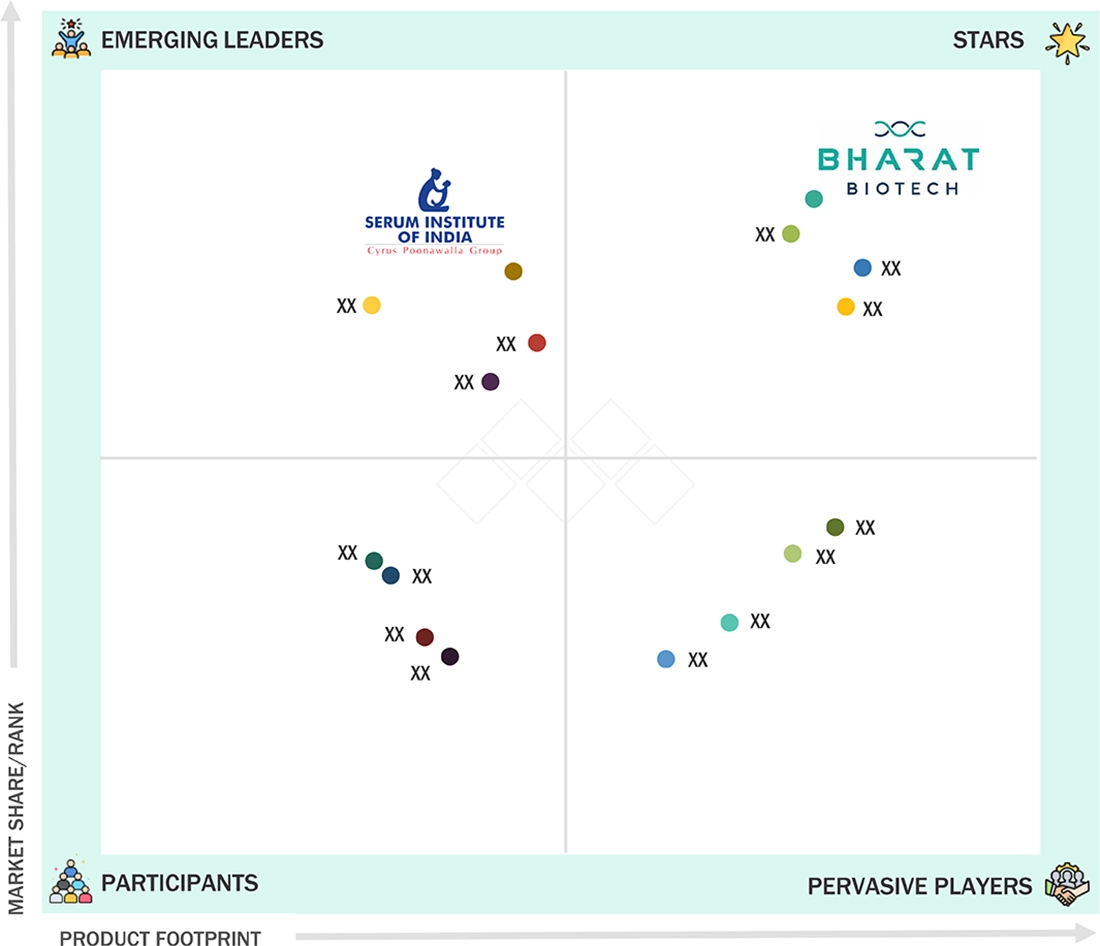
COMPETITIVE LANDSCAPESerum Institute of India (India), Pfizer Inc. (US), and Merck & Co. (US) were identified as the star players in the Asia Pacific vaccines market, given their strong market share and product footprint.
-
COMPETITIVE LANDSCAPESinovac (China) and Indian Immunologicals Limited (India), among others, have distinguished themselves among start-ups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
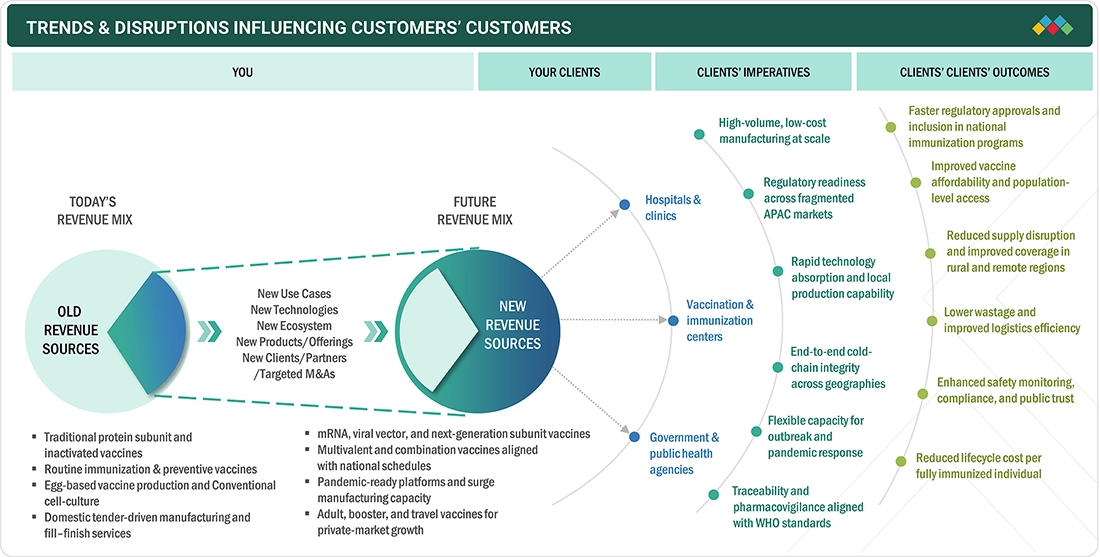
The Asia Pacific vaccines market is growing quickly, fueled by strong research investments, active public-private partnerships, and greater use of vaccines in preventive healthcare and clinical decision-making. Advanced vaccine platforms allow researchers and clinicians to better understand infectious diseases, identify new therapeutic targets, and develop more precise immunization approaches.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The Asia Pacific vaccines market is experiencing a transformation driven by rapidly changing scientific, regulatory, and clinical developments, with trends expected to grow stronger during the forecast period. Increasing use of vaccines for targeted immunization against infectious diseases, cancer treatments, and emerging pathogens, supported by vigorous clinical trial activity and public health initiatives, boosts demand for scalable, dependable platforms across hospitals and research institutions.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Ongoing advancements in vaccine development technologies

-
Rising healthcare expenditure
Level
-
Cold chain and distribution infrastructure gaps
Level
-
Rapid adoption of advanced technologies and platforms
Level
-
Uneven healthcare infrastructure development
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Ongoing advancements in vaccine development technologies
Technological advancements in vaccine development, including mRNA and DNA vaccines, drive the Asia Pacific vaccines market forward. These platforms enable faster development timelines and higher efficacy against complex pathogens like dengue, tuberculosis, and influenza strains endemic to the region.
Restraint: Cold chain and distribution infrastructure gaps
Gaps in cold chain and distribution infrastructure, especially in rural and low-income communities, hinder proper vaccine delivery and storage. Many of these areas lack reliable electricity, adequate refrigeration, and temperature-controlled transport, which can cause vaccines to spoil during transit or storage.
Opportunity: Rapid adoption of advanced technologies and platforms
Adoption of advanced vaccine technologies such as mRNA, viral vectors, and DNA platforms in Asia Pacific opens up opportunities for tailored solutions that target regional disease burdens, including dengue, tuberculosis, and emerging zoonotic diseases common in densely populated areas.
Challenge: Uneven healthcare infrastructure development
Uneven healthcare infrastructure across Asia Pacific countries creates significant barriers to equitable vaccine access and immunization coverage. Rural and remote areas often lack sufficient clinics, trained personnel, and cold chain logistics, resulting in lower vaccination rates compared to urban centers.
ASIA PACIFIC VACCINES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Advanced mRNA vaccine technology and manufacturing for large-scale production using biopharma platforms for infectious diseases and public health programs | Improves robust vaccine supply and deployment for clinical trials and global immunization efforts in Asia Pacific, leveraging Pune's biotech hub for cost-effective, high-volume output |
 |
High-throughput vaccine platforms integrating advanced adjuvants like Algel-IMDG for discovery and broad-spectrum coverage against emerging pathogens | Delivers strong immunogenicity, aiding regulatory approvals and generating data for public health campaigns in India and beyond |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Asia Pacific vaccines market functions through a closely connected network involving instrument providers, bioinformatics firms, contract research organizations (CROs), biopharmaceutical developers, and clinic-based facilities. These partnerships enable high-throughput manufacturing and analytical platforms for vaccines, while research-driven trials carried out in CROs and core labs support hospitals and biopharma companies in assessing vaccine effectiveness.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Asia Pacific Vaccines Market, By Disease Indication
In 2024, the pneumococcal diseases segment held the largest share in the Asia Pacific vaccines market, driven by high pneumococcal infection rates, increased government funding for immunization programs, and improved public awareness of serious complications from these diseases.
Asia Pacific Vaccines Market, By Type
In 2024, the multivalent vaccines segment led the Asia Pacific vaccines market, mainly because they can simplify immunization schedules and provide comprehensive protection in a single dose.
Asia Pacific Vaccines Market, By Technology
Conjugate vaccine technology holds the largest market share in Asia Pacific due to its ability to generate strong, long-lasting immunity by linking weak polysaccharide antigens to effective protein carriers, which elicits powerful T-cell responses and lasting immunologic memory.
Asia Pacific Vaccines Market, By Route of Administration
The intramuscular & subcutaneous segment led the Asia Pacific vaccines market, as this method ensures swift, dependable absorption for a strong and predictable immune response.
Asia Pacific Vaccines Market, By End User
Adult vaccines held a larger market share due to increasing demand for boosters against shingles, pneumococcal disease, and influenza. Government-supported campaigns targeting working adults and seniors, along with expanded private immunization efforts in urban India and Southeast Asia, further drive this dominance.
REGION
China to be fastest-growing market during forecast period
China is the fastest-growing market for vaccines in Asia Pacific, propelled by ongoing expansion of commercial vaccine production capacity. Robust pipelines in mRNA vaccines, next-generation influenza vaccines, and advanced modalities, along with significant investments in large CDMO campuses and facility upgrades by major companies like Sinovac and Walvax, have accelerated the adoption of high-performance manufacturing lines across new and existing plants.

ASIA PACIFIC VACCINES MARKET: COMPANY EVALUATION MATRIX
Serum Institute of India (Star) has large-scale vaccination operations through its significant manufacturing capacity, cost-effective strategies, and strong partnerships with governments and procurement authorities for vaccinations. The company’s diverse portfolio of pediatric, pandemic, and combination vaccines forms the foundation for the public sector’s vaccination plans. Bharat Biotech (Emergent Leader) is positioning itself through innovation platforms such as indigenous vaccine development, viral vector technologies, and expanded R&D efforts in developing next-generation and neglected vaccines. This complements the growing presence of regional vaccine manufacturers in the Asian market, known for their expertise in affordable formulations and potential for rapid scale-up.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- SERUM INSTITUTE OF INDIA (INDIA)
- Pfizer Inc. (US)
- Merck & Co., Inc. (US)
- GSK Plc. (UK)
- BHARAT BIOTECH (INDIA)
- PANACEA BIOTEC (INDIA)
- BIOLOGICAL E LIMITED (INDIA)
- MITSUBISHI TANABE PHARMA CORPORATION (JAPAN)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 9.46 BN |
| Market Forecast in 2030 (Value) | USD 14.57 BN |
| Growth Rate | CAGR of 9.0% from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD MN/BN) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered | By Disease Indication: Pneumococcal Diseases, Influenza, Combination Vaccines, HPV, Meningococcal Diseases, Herpes Zoster, Rotavirus, MMR, Varicella, Hepatitis, DTP, Polio, RSV, COVID-19, Other Disease Indications I By Technology: Conjugate Vaccines, Inactivated & Subunit Vaccines, Live Attenuated Vaccines, Recombinant Vaccines, Toxoid Vaccines, Other Vaccines I By Type: Monovalent Vaccines, Multivalent Vaccines I By Route of Administration: Intramuscular & Subcutaneous, Oral, Other Routes of Administration I By End User: Pediatric Vaccines, Adult Vaccines |
| Countries Covered | China, Japan, India, South Korea, Rest of Asia Pacific |
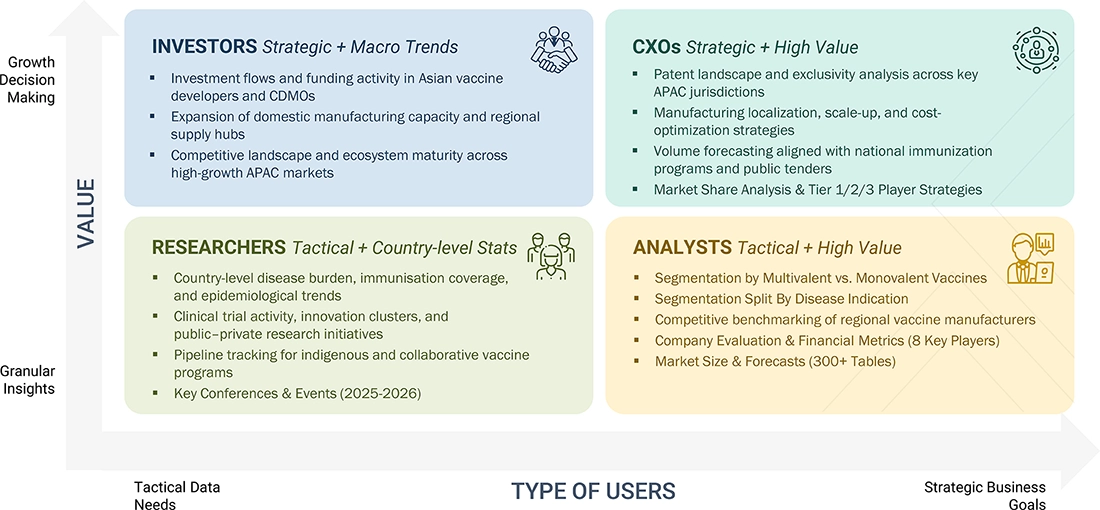
WHAT IS IN IT FOR YOU: ASIA PACIFIC VACCINES MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Regional Partnerships with Vaccine Manufacturers | Structured collaboration models with regional and domestic vaccine manufacturers, including localized manufacturing partnerships, fill–finish alliances, and technology transfer programs aligned with Asia Pacific regulatory and public procurement requirements. | Accelerates market entry across high-growth Asia Pacific countries, strengthens supply security, improves cost competitiveness, and supports participation in national immunization tenders and multilateral procurement programs. |
| Regulatory, Clinical, and Market Access Enablement | Asia Pacific-specific clinical development strategies, regulatory pathway optimization across heterogeneous markets, and market access planning aligned with government immunization schedules and public–private distribution models. | Reduces approval timelines, enhances alignment with national vaccination priorities, improves inclusion in public immunization programs, and drives sustainable uptake across diverse Asia Pacific populations. |
RECENT DEVELOPMENTS
- January 2025 : Primrose Bio, Inc. and Serum Institute of India (SII) have entered into a collaboration to develop a novel multi-antigen vaccine. Primrose will contribute its proprietary Pfenex Expression Technology for manufacturing strain development, while SII will lead preclinical and clinical development and handle commercialization of the first indigenously developed qHPV vaccine, CEREVAC.
- April 2024 : Bharat Biotech and Bilthoven Biologicals B.V., a wholly owned subsidiary of the Serum Institute of India, collaborated to strengthen the production and supply security of oral polio vaccines (OPV).
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the Asia Pacific vaccines market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive technical, market-oriented, and commercial study of the Asia Pacific vaccines market. The secondary sources used for this study include World Health Organization (WHO), the Organization for Economic Co-operation and Development (OECD), National Center for Biotechnology Information (NCBI), Centers for Disease Control and Prevention (CDC), the Global Cancer Observatory (GLOBOCAN), the National Institutes of Health (NIH), Center of Disease Control & Prevention (CDC), US Department of Health and Human Services, National Institutes of Health (NIH), National Library of Medicine, National Center for Biotechnology Information (NCBI), National Institute of Allergy and Infectious Diseases (NIAID), World Cancer Research Fund International (WCRF International), European Medicines Agency (EMA), The National Medical Products Administration (NMPA), Global Alliance for Vaccines and Immunization (GAVI), United States Food & Drug Administration (US FDA), Orange book, Purple book, Clinical trials.gov, Pan American Health Organization (PAHO), United Nation International Children’s Emergency Fund (UNICEF), Department of health and Human Services (HHS), and International Society for Vaccines (ISV). Corporate filings include annual reports, SEC filings, investor presentations, and financial statements; research journals; press releases; and trade, business, and professional associations. Secondary data was collected and analyzed to arrive at the overall size of the Asia Pacific vaccines market, which was validated through primary research. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were employed to estimate and validate the overall size of the Asia Pacific vaccines market. These methods were also widely used to determine the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:

Data Triangulation
After estimating the overall market size through the market size estimation process, the total market was divided into several segments and subsegments. Data triangulation and market breakdown techniques were used where applicable to finalize the overall market analysis and obtain precise statistics for all segments and subsegments. The data was triangulated by examining various factors and trends from both demand and supply sides.
Market Definition
A vaccine is a biologically formulated product designed to trigger active acquired immunity against a specific infectious or malignant disease. Vaccines work by stimulating the immune system to identify and fight harmful agents, such as viruses or bacteria. They generally consist of parts that resemble the disease-causing microorganism, often in weakened or deactivated forms, along with their toxins or surface proteins. The report solely focuses on human vaccines and does not include veterinary vaccines, which are outside the scope of this study.
Stakeholders
- Vaccine product manufacturers and suppliers
- Distributors and suppliers of vaccine products
- Vaccine research institutes
- Biotechnology and biopharmaceutical companies
- Contract manufacturing organizations (CMOs)
- Contract development and manufacturing organizations (CDMO)
- Suppliers and distributors of pharmaceutical products
- Research and development (R&D) companies
- Drug Manufacturers, Vendors, and Distributors
- Immunization centres
- Hospitals and laboratories
- Trade associations and industry bodies
- Regulatory bodies and government organizations
- Venture capitalists and investors
- Hospitals
- Specialty Clinics
Report Objectives
- To define, describe, and forecast the Asia Pacific vaccines market by technology, type, disease indication, route of administration, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall Asia Pacific vaccines market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders
- To profile the key players and analyze their market shares and core competencies
- To track and analyze competitive developments, such as product launches, agreements, partnerships, acquisitions, regulatory approvals, and research & development activities
- To analyze and provide funding & investment activities, brand/product comparative analysis, and vendor valuation & financial metrics of the Asia Pacific vaccines market.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Asia Pacific Vaccines Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Asia Pacific Vaccines Market