
North America Vaccines Market Size, Growth, Share & Trends Analysis
North America Vaccines Market by Technology (Conjugate, Recombinant, Inactivated, Live Attenuated, Viral Vector), Type (Monovalent, Multivalent), Disease (Pneumococcal, Flu, Hepatitis, MMR), Route of Administration (IM, SC, Oral) - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
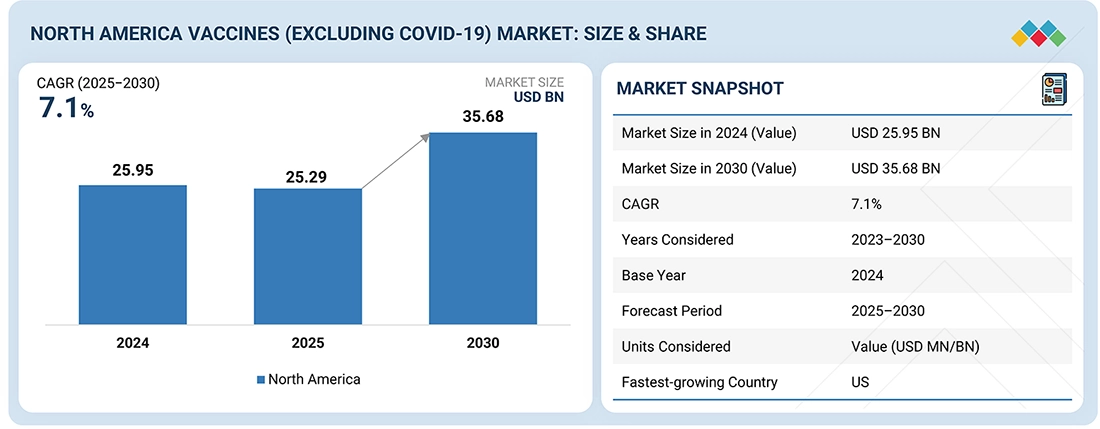
The North America vaccines market, valued at US$25.95 billion in 2024, stood at US$25.29 billion in 2025 and is projected to advance at a resilient CAGR of 7.1% from 2025 to 2030, culminating in a forecasted valuation of US$35.68 billion by the end of the period. The vaccines market is growing rapidly, driven by swift global vaccine development and commercialization, higher rates of infectious diseases necessitating preventive vaccines, expanded immunization initiatives, innovations in vaccine technology, and increased government funding and investments for novel vaccines against diverse diseases.
KEY TAKEAWAYS
-
BY COUNTRYThe US vaccines market is projected to be the fastest-growing segment with a CAGR of 7.3% from 2025 to 2030.
-
BY DISEASEPneumococcal disease dominated the market with a 94.6% share, driven by the high incidence of disease and rising investments in vaccination programs.
-
BY TECHNOLOGYConjugate vaccines had the highest share at 94.4%, driven by increased government support and rising public-private partnerships for their development.
-
BY TYPEThe multivalent vaccines segment had the highest share, mainly due to its cost-effectiveness and the increasing need for immunization and eradication of infectious diseases.
-
COMPETITIVE LANDSCAPE: Key PlayersPfizer (US), GSK (UK), and Merck & Co (US) were identified as some of the star players in the North American vaccines market, given their strong market share and product portfolios.
-
COMPETITIVE LANDSCAPE: Start-upsInovio Pharmaceuticals (US) and VBI Vaccines (US), among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The North America vaccines market is expanding steadily, supported by strong research funding, robust public-private partnerships, and growing use of vaccines in preventive healthcare and clinical decision-making. Advanced vaccine platforms help researchers and clinicians better understand infectious disease biology, identify new therapeutic targets, and develop more precise immunization strategies.
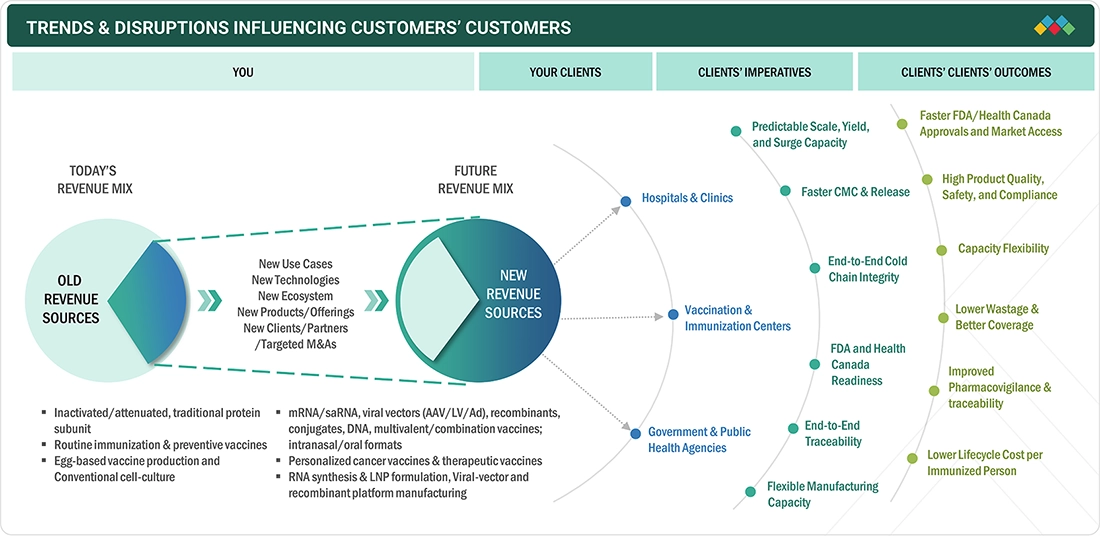
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The North America vaccines market is undergoing a transformation driven by rapidly evolving scientific, regulatory, and clinical developments, trends expected to intensify through the forecast horizon. The increasing adoption of vaccines for targeted immunization against infectious diseases, oncology applications, and novel pathogens, paired with robust clinical trial activity and public health efforts, fuels demand for scalable, reliable platforms across hospitals and research institutions.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
High prevalence of infectious diseases and preventive healthcare emphasis

-
Technological advancement in vaccine development
Level
-
High development and production costs
Level
-
Innovative vaccine platforms and combination vaccines
Level
-
Regulatory and approval complexities
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: High prevalence of infectious diseases and preventive healthcare emphasis
Rising cases of seasonal and emerging infections (e.g., influenza, HPV) drive sustained demand for immunization. Strong awareness of vaccine benefits and preventive healthcare programs supports adoption across age groups.
Restraint: High development and production costs
Vaccine R&D involves significant capital, lengthy clinical trials, and complex manufacturing processes, thereby increasing overall costs for companies and potentially for payers or patients.
Opportunity: Innovative vaccine platforms and combination vaccines
Continued development of next-generation technologies (e.g., mRNA beyond COVID-19, combination vaccines) can open new therapeutic indications and increase uptake due to convenience and broader protection.
Challenge: Regulatory and approval complexities
Tightening regulatory scrutiny and potential policy shifts (e.g., evolving CDC recommendations or FDA oversight) may create uncertainty and delay product launches.
NORTH AMERICA VACCINES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Advanced vaccine platforms, mRNA technologies, and manufacturing solutions for large-scale production used by biopharma firms and public health programs? | Improves robust vaccine validation and deployment for infectious diseases and oncology, supporting clinical trials and global immunization efforts ? |
 |
High-throughput vaccine solutions like mRNA and subunit platforms, aiding biopharma discovery and patient access in North America? | Delivers insightful immunogenicity profiles, helping qualification for regulatory approvals & generating data for public health campaigns |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The North American market of vaccines is integrated in a closely networked fashion between companies dealing in instruments, bioinformatics companies, contract research organizations, bio pharmaceutical developers, and clinic-based facilities. They provide high-throughput manufacturing platforms as well as analysis platforms for vaccines, whereas research-driven trials are carried out in contract research organizations, core facilities, so, in effect, they assist hospitals, as well as bio pharmaceutical companies, in determining the efficiency of a vaccine.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
North America Vaccines Market, By Disease Indication
In 2024, the pneumococcal segment held the largest share due to several key factors like rising incidence of pneumococcal infections, increased government funding for vaccination programs and heightened public awareness of the serious health risks associated with pneumococcal-related complications.
North America Vaccines Market, By Type
In 2024, the multivalent vaccines segment held the largest share of the vaccines market. This is mainly because they can simplify immunization programs by providing broad protection in a single formulation.
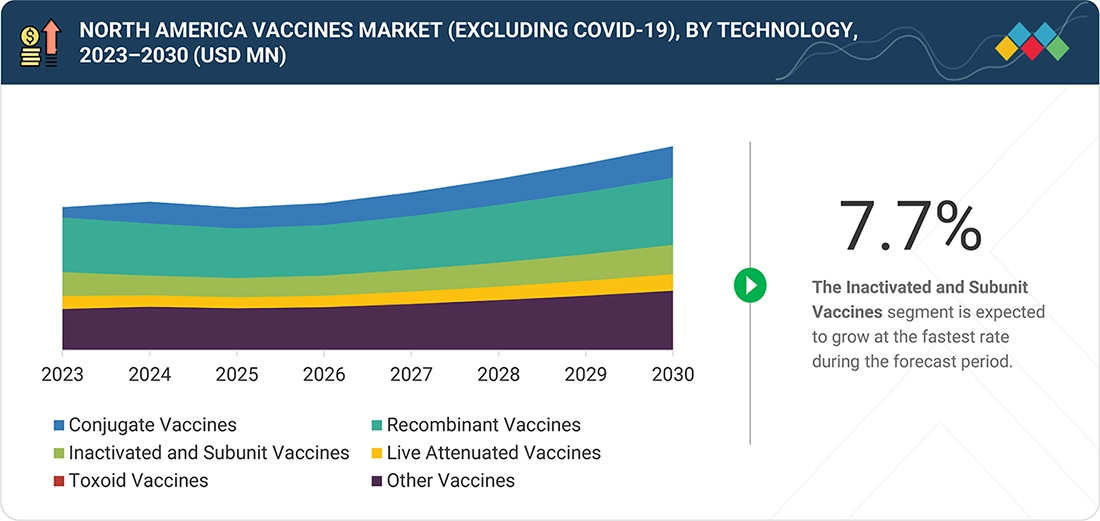
North America Vaccines Market, By Technology
The conjugate vaccine technology holds the largest market share. This is primarily due to their ability to generate strong, long-lasting immunity by linking weak polysaccharide antigens to potent protein carriers, thereby eliciting powerful T-cell-dependent responses and establishing lasting immunologic memory.
North America Vaccines Market, By Route of Administration
The intramuscular and subcutaneous routes segment accounted for the largest share of the market. This is because they facilitate rapid, reliable absorption, resulting in robust, consistent immune responses.
North America Vaccines Market, By End User
Adult end users account for the largest share in the vaccines market due to the rising incidence of infectious diseases among adults and the significant increase in the aging population, which is more susceptible to severe outcomes from infections.
REGION
The US is projected to be the fastest-growing country in the market during the forecast period.
The US is projected to remain one of the fastest-growing vaccine markets in North America, driven by the ongoing expansion of commercial vaccine production capacity. Strong pipelines in mRNA vaccines, next-generation influenza shots, and advanced modalities, coupled with substantial investments in large CDMO campuses and facility upgrades by major pharma companies, accelerated the adoption of high-performance manufacturing trains across new and existing plants.
NORTH AMERICA VACCINES MARKET: COMPANY EVALUATION MATRIX
In the North America vaccines market matrix, Merck (Star Player) has widely adopted mRNA platforms, viral vector technologies, and combination vaccines, which anchor major biopharma, CRO, and public health workflows. CSL (Emerging Leader) is building share with its protein subunit and adjuvanted solutions that support targeted immunization and next-generation vaccines. Smaller specialist vaccine developers focused on niche pathogens and agile manufacturing add further competitive momentum.
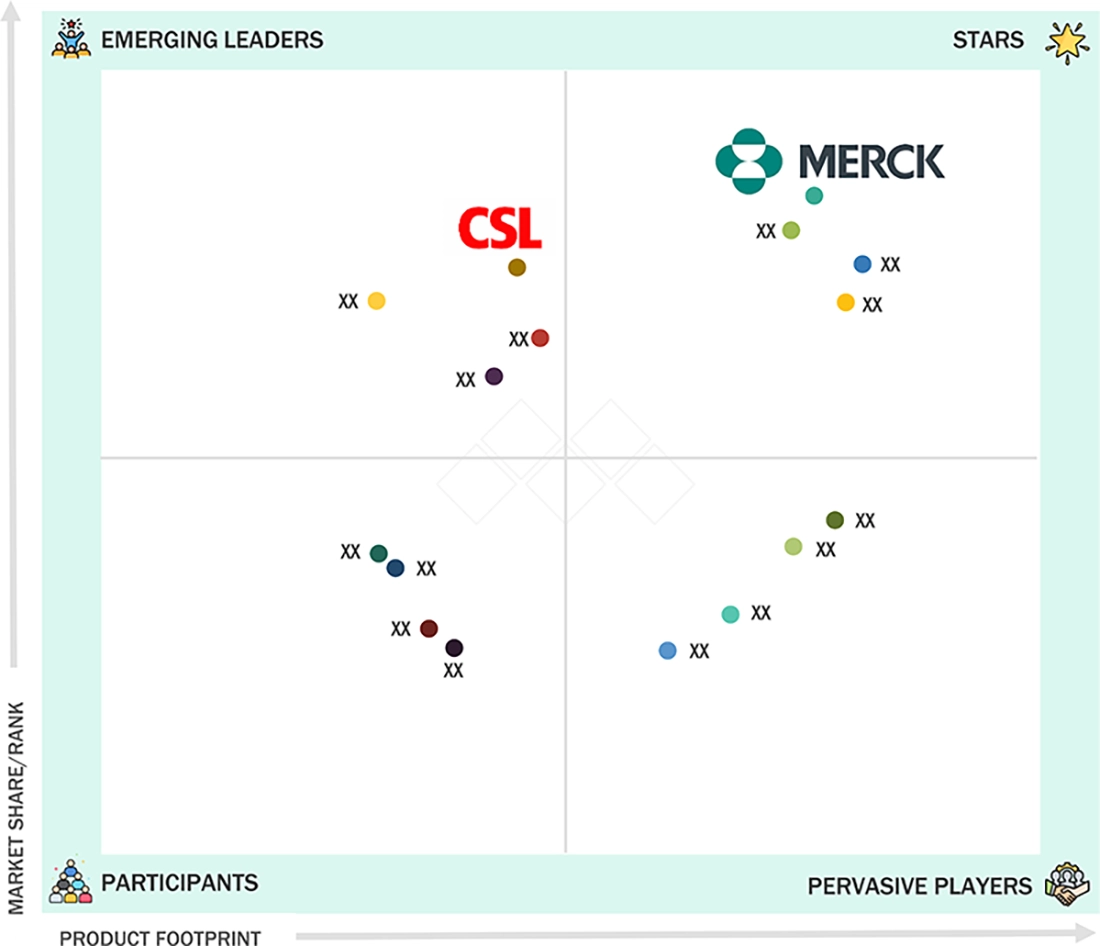
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Merck & Co., Inc. (US)
- Pfizer, Inc. (US)
- GSK Plc. (UK)
- Johnson & Johnson Services, Inc. (US)
- Sanofi (France)
- CSL (Australia)
- Emergent BioSolutions (US)
- Novavax, Inc. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 25.95 Billion |
| Market Size in 2030 (Value) | USD 35.68 Billion |
| Growth Rate | CAGR of 7.1 % from 2025 to 2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered | By Disease Indication: Pneumococcal disease, influenza, combination vaccines, HPV, meningococcal disease, herpes zoster, rotavirus,MMR, varicella, hepatitis, DTP, polio, RSV, COVID-19, other disease indications I By Technology: Conjugate vaccines, inactivated & subunit vaccines, live attenuated vaccines, recombinant vaccines, toxoid vaccines, and other vaccines I By Type: Monovalent , multivalent I By Route of Administration: Intramuscular & subcutaneous administration, oral administration, and other routes of administration I By End User: Pediatric vaccines and adult vaccines |
| Countries Covered | US and Canada |
| Parent & Related Segment Reports |
Vaccines Market Conjugate Vaccines Market Europe Vaccines Market Respiratory Syncytial Virus (RSV) Vaccines Market Asia Pacific Vaccines Market |
WHAT IS IN IT FOR YOU: NORTH AMERICA VACCINES MARKET REPORT CONTENT GUIDE
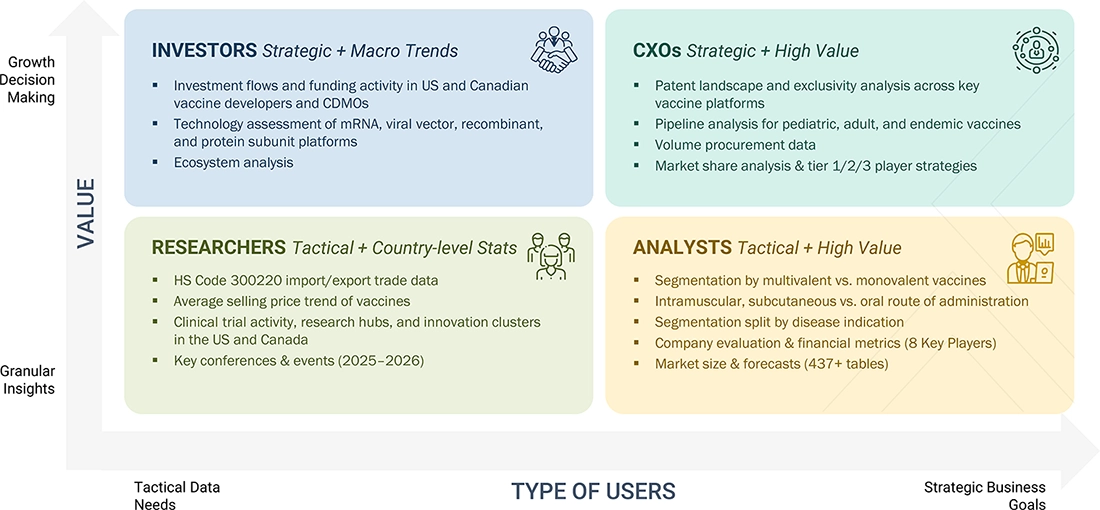
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Strategic Partnerships with Vaccine Manufacturers | Customized engagement models for collaborations with large pharma and emerging biotech players, including co-development, contract manufacturing, and technology transfer initiatives across North America. | Enables rapid pipeline expansion, de-risked development, and access to advanced technologies while improving commercialization success in the US and Canadian markets |
| Clinical and Commercial Optimization | Optimized clinical trial designs, real-world evidence generation, and commercialization strategies tailored to North American immunization programs and reimbursement structures. | Improves approval success rates, enhances payer and public health acceptance, and supports sustained vaccine uptake across priority population segments |
RECENT DEVELOPMENTS
- February 2024 : Pfizer released positive top-line data on the season two efficacy of ABRYSVO, a bivalent vaccine, for RSV in adults 60 and older.
- September 2023 : Merck & Co., Inc. (US) received approval from the European Commission (EC) for an expanded indication for ERVEBO [Ebola Zaire Vaccine], for active immunization of individuals one year of age or older to protect against EVD caused by the Zaire ebolavirus.
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the North America vaccines market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive technical, market-oriented, and commercial study of the North America vaccines market. The secondary sources used for this study include World Health Organization (WHO), the Organization for Economic Co-operation and Development (OECD), National Center for Biotechnology Information (NCBI), Centers for Disease Control and Prevention (CDC), the Global Cancer Observatory (GLOBOCAN), the National Institutes of Health (NIH), Center of Disease Control & Prevention (CDC), US Department of Health and Human Services, National Institutes of Health (NIH), National Library of Medicine, National Center for Biotechnology Information (NCBI), National Institute of Allergy and Infectious Diseases (NIAID), World Cancer Research Fund International (WCRF International), European Medicines Agency (EMA), The National Medical Products Administration (NMPA), Global Alliance for Vaccines and Immunization (GAVI), United States Food & Drug Administration (US FDA), Orange book, Purple book, Clinical trials.gov, Pan American Health Organization (PAHO), United Nation International Children’s Emergency Fund (UNICEF), Department of health and Human Services (HHS), and International Society for Vaccines (ISV). Corporate filings include annual reports, SEC filings, investor presentations, and financial statements; research journals; press releases; and trade, business, and professional associations. Secondary data was collected and analyzed to arrive at the overall size of the North America vaccines market, which was validated through primary research. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information.
Market Size Estimation
Both top-down and bottom-up approaches were employed to estimate and validate the overall size of the vaccines market. These methods were also widely used to determine the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Data Triangulation
After estimating the overall market size through the market size estimation process, the total market was divided into several segments and subsegments. Data triangulation and market breakdown techniques were used where applicable to finalize the overall market analysis and obtain precise statistics for all segments and subsegments. The data was triangulated by examining various factors and trends from both demand and supply sides.
Market Definition
A vaccine is a biologically formulated product designed to trigger active acquired immunity against a specific infectious or malignant disease. Vaccines work by stimulating the immune system to identify and fight harmful agents, such as viruses or bacteria. They generally consist of parts that resemble the disease-causing microorganism, often in weakened or deactivated forms, along with their toxins or surface proteins. The report solely focuses on human vaccines and does not include veterinary vaccines, which are outside the scope of this study.
Stakeholders
- Vaccine product manufacturers and suppliers
- Distributors and suppliers of vaccine products
- Vaccine research institutes
- Biotechnology and biopharmaceutical companies
- Contract manufacturing organizations (CMOs)
- Contract development and manufacturing organizations (CDMO)
- Suppliers and distributors of pharmaceutical products
- Research and development (R&D) companies
- Drug Manufacturers, Vendors, and Distributors
- Immunization centres
- Hospitals and laboratories
- Trade associations and industry bodies
- Regulatory bodies and government organizations
- Venture capitalists and investors
- Hospitals
- Specialty Clinics
Report Objectives
- To define, describe, and forecast the vaccines market by technology, type, disease indication, route of administration, and end user
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall vaccines market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders
- To profile the key players and analyze their market shares and core competencies2
- To track and analyze competitive developments, such as product launches, agreements, partnerships, acquisitions, regulatory approvals, and research & development activities
- To analyze and provide funding & investment activities, brand/product comparative analysis, and vendor valuation & financial metrics of the vaccines market.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the North America Vaccines Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in North America Vaccines Market