
Europe Vaccines Market Size, Growth, Share & Trends Analysis
Europe Vaccines Market by Technology (Conjugate, Recombinant, Inactivated, Live Attenuated, Viral Vector, mRNA), Type (Monovalent, Multivalent), Disease (Pneumococcal, Flu, Hepatitis, MMR, RSV), Route of Administration (IM, SC, Oral) - Forecast to 2030




OVERVIEW
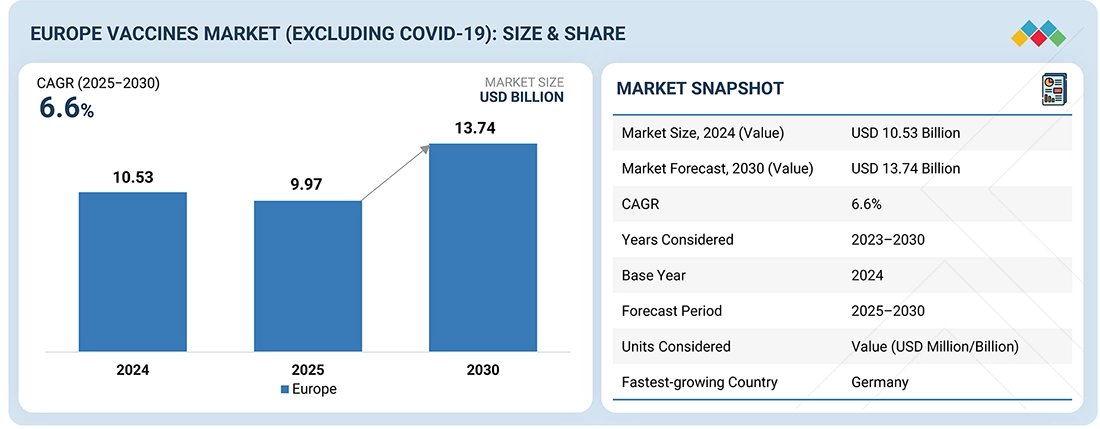
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The Europe vaccines market is projected to reach USD 13.74 billion by 2030 from USD 9.97 billion in 2025, at a CAGR of 6.6%. The vaccines market is growing due to fast global developments and the commercialization of vaccines, rising rates of infectious diseases, which require preventive measures, and government initiatives to encourage vaccinations, immunization programs, advancements, and investments in new vaccines against various diseases.
KEY TAKEAWAYS
-
BY COUNTRYThe German vaccines market is projected to be the fastest-growing country segment at a CAGR of 8.0%.
-
BY DISEASEPneumococcal disease commanded the largest share of 29.1% in this segment, driven by escalating disease incidence across populations.
-
BY TECHNOLOGYConjugate vaccines captured the highest share of 32%, fueled by growing government backing and expanding public-private collaborations for their advancement.
-
BY TYPEMultivalent vaccines led by share, primarily due to their economic advantages and surging demand for comprehensive immunization against infectious diseases.
-
COMPETITIVE LANDSCAPEGSK (UK) and Sanofi (US) were identified as some of the star players in the Europe vaccines market, given their strong market share and product footprint.
-
COMPETITIVE LANDSCAPEValneva SE (France) and Microgen (Russia), among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The Europe vaccines market expands steadily, bolstered by substantial research funding, robust public-private partnerships, and increasing integration of vaccines into preventive healthcare and clinical decision-making. Advanced vaccine platforms empower researchers and clinicians to deepen understanding of infectious disease biology, pinpoint novel therapeutic targets, and craft precision immunization strategies.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The Europe vaccines market is undergoing a transformation from rapidly evolving scientific, regulatory, and clinical developments, trends are expected to rise through the forecast horizon. Increasing adoption of vaccines for targeted immunization against infectious diseases, oncology applications, and novel pathogens paired with robust clinical trial activity and public health efforts fuel demand for scalable, dependable platforms across hospitals and research institutions.
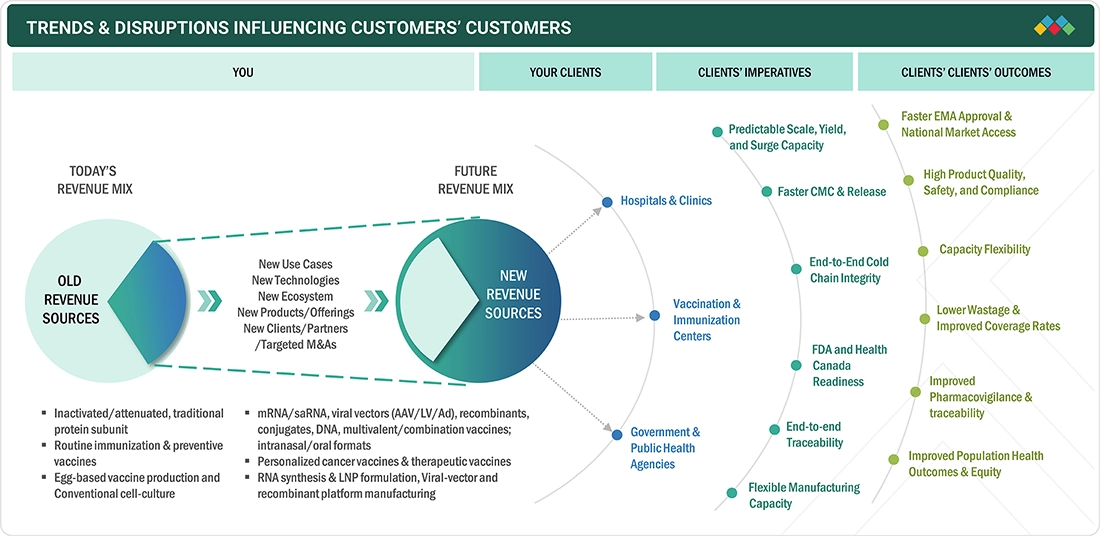
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
High Disease Surveillance and Preventive Healthcare Orientation

-
Aging Population and Adult Immunization Expansion
Level
-
Pricing and Reimbursement Pressure
Level
-
Next-Generation and Combination Vaccines
Level
-
Intense Competition Among Multinational Players
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: High Disease Surveillance and Preventive Healthcare Orientation
Europe’s strong public health infrastructure is vital for effective vaccination policies. This system enhances vaccine uptake and allows for the rapid implementation of new vaccine formulations in response to health threats. With coordinated healthcare systems and organizations, Europe can quickly share information, mobilize resources, and launch vaccination campaigns. This adaptability helps tackle public health challenges, ensuring timely immunization against diseases. By prioritizing vaccination, Europe shows its commitment to protecting public health and building community resilience.
Restraint: Pricing and Reimbursement Pressure
Centralized procurement and tender-based purchasing methods often impose rigid pricing structures, which can significantly restrict pricing flexibility in the marketplace. This is particularly evident in the realm of mature vaccines, where competition and innovation may be stifled by these procurement practices. As a result, companies are often forced to operate within narrow margins, which can diminish their incentive to invest in research and development or to enhance the quality of their products. The constrained pricing environment can also lead to challenges in sustaining operations and may limit the availability of vaccines in certain regions. Ultimately, while these procurement models aim to ensure cost-effectiveness and equitable access, they can inadvertently compress the financial viability of producers, potentially impacting the overall health infrastructure and vaccine availability.
Opportunity: Next-Generation and Combination Vaccines
As the landscape of healthcare evolves, there is a growing demand for broader protection against various infectious diseases, particularly through the use of combination and multivalent vaccines. These vaccines allow for the simultaneous targeting of multiple pathogens, thereby reducing the need for numerous individual injections. This not only enhances patient compliance by minimizing the number of visits to healthcare providers but also optimizes the immune response by presenting a wider array of antigens. Ultimately, the push for combination and multivalent vaccines reflects a desire for more efficient vaccination strategies that provide comprehensive protection while making the immunization process more convenient and accessible for individuals of all ages.
Challenge: Intense Competition Among Multinational Players
The pharmaceutical market is significantly influenced by a few large companies, such as GSK, Sanofi, Pfizer, and Moderna, which hold substantial incumbency advantages in various aspects of the industry. These advantages stem from their extensive resources, established brand recognition, and robust distribution networks, making it challenging for smaller or emerging companies to compete effectively. Furthermore, these dominant players benefit from economies of scale, allowing them to invest heavily in research and development, marketing, and regulatory compliance. As a result, they are often first to market with innovative treatments and vaccines, solidifying their market position and customer loyalty. This environment creates high barriers to entry for new competitors, while also raising concerns about pricing and access to medications for consumers.
EUROPE VACCINES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Advanced vaccine platforms and manufacturing solutions for infectious diseases, supporting clinical trials and European immunization programs | Enhanced immunogenicity profiles, regulatory approvals, and generating data for public health campaigns ? |
 |
High-throughput vaccine solutions like mRNA technologies for infectious disease prevention and deployment | Improved vaccine validation, supporting clinical and global immunization efforts in Europe |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe market of vaccines is integrated in a closely connected network between companies dealing in instruments, bioinformatics companies, contract research organizations, biopharmaceutical developers, and clinic-based facilities. They provide high-throughput manufacturing platforms as well as analysis platforms for vaccines, whereas research-driven trials are carried out in contract research organizations, core facilities, so, in effect, they assist hospitals, as well as biopharmaceutical companies, in determining the efficiency of a vaccine.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
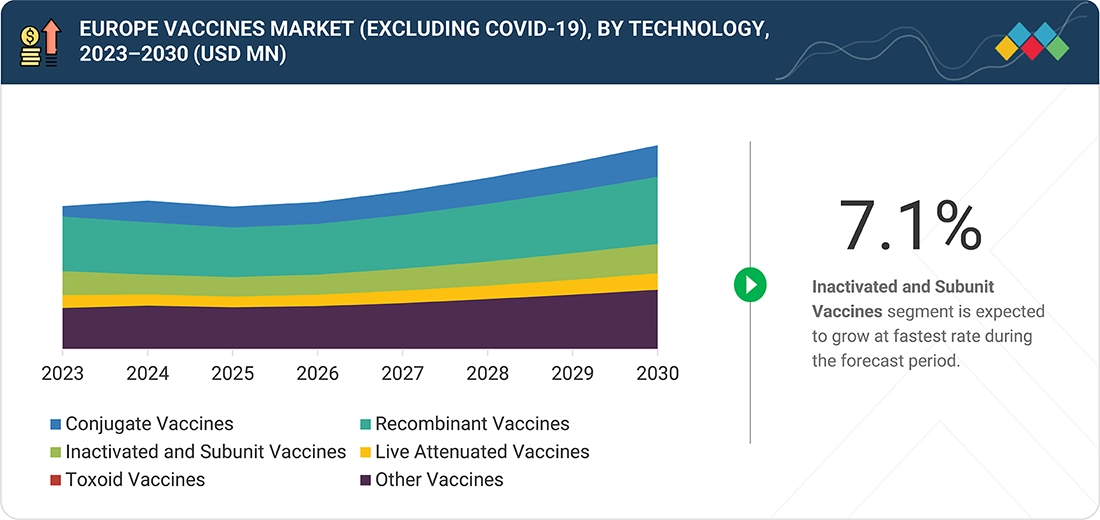
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe Vaccines Market, By Disease Indication
In 2024, the pneumococcal segment held the largest share due to rising pneumococcal infection rates driving national vaccination programs, alongside heightened public funding addressing serious health risks from pneumococcal complications.
Europe Vaccines Market, By Type
In 2024, multivalent vaccines commanded the largest share in the Europe vaccines market by providing broad protection through single formulations for immunization schedules.
Europe Vaccines Market, By Technology
Conjugate vaccines hold the largest segment due to their superior ability to generate strong, long-lasting T-cell immunity by eliciting potent T-cell-dependent responses and establishing lasting immunological memory.
Europe Vaccines Market, By Route of Administration
Subcutaneous routes account for the largest share as this segment facilitates rapid and reliable absorption, resulting in robust and consistent immune responses.
Europe Vaccines Market, By End User
Adult end users represent the largest share in the Europe vaccines market due to the aging population and rising susceptibility to severe infection outcomes.
REGION
Germany to be the fastest-growing country during the forecast period
During the forecast period, Germany remained one of the fastest-growing markets for vaccines in Europe, driven by the continuous expansion of commercial vaccine production capacity. Strong pipelines in mRNA vaccines, next-generation influenza formulations, and advanced modalities, combined with substantial investments in large CDMO campuses and facility upgrades by leading pharma companies, have accelerated the adoption of high-performance manufacturing trains across new and existing plants.
EUROPE VACCINES MARKET: COMPANY EVALUATION MATRIX
In the Europe vaccines market matrix, GSK (Star Player) dominates with widely adopted viral vector platforms, adjuvanted technologies, and multivalent vaccines anchoring biopharma production, clinical trials, and national immunization programs across the continent. Bavarian Nordic (Emerging Leader) builds market share through innovative poxvirus vectors and protein-based solutions supporting targeted outbreak response vaccines and next-generation platforms.
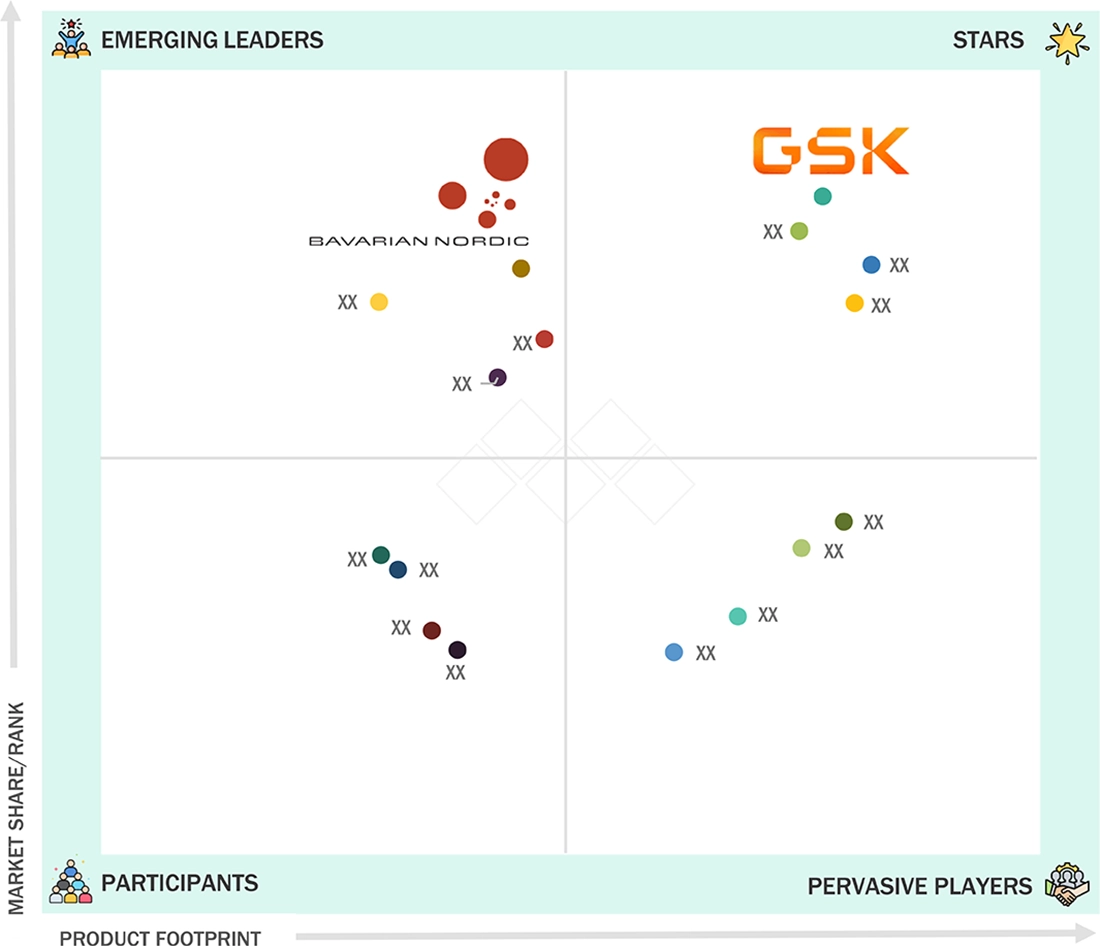
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- GSK Plc. (UK)
- Sanofi (France)
- BAVARIAN NORDIC (Denmark)
- ASTRAZENECA PLC (UK)
- CSL (Australia)
- Pfizer (US)
- MERCK & CO., INC (US)
- JOHNSON & JOHNSON SERVICES, INC. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size, 2024 (Value) | USD 10.53 Billion |
| Market Forecast, 2030 (Value) | USD 13.74 Billion |
| Growth Rate | CAGR of 6.6% from 2025 to 2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
|
| Countries Covered | Germany, UK, France, Italy, Spain, Rest of Europe |
WHAT IS IN IT FOR YOU: EUROPE VACCINES MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Strategic partnerships customized engagement models for collaborations with large pharma and biotech | Flexible, rapid expansion, and de-risked development access to European markets | Improved regulatory success rates, public health impact |
| Clinical & commercial optimization tested in real-world evidence generation and reimbursement structures | Superior clinical outcomes, priority patient access | Supported scale-up priority segments ? |
RECENT DEVELOPMENTS
- February 2025 : GSK announced the US Food and Drug Administration (FDA) approval for Penmenvy (Meningococcal Groups A, B, C, W, and Y Vaccine) for use in individuals aged 10 through 25 years. The vaccine targeted five major serogroups of Neisseria meningitidis (A, B, C, W, and Y), which commonly cause invasive meningococcal disease (IMD).
- July 2025 : Sanofi announced an agreement to acquire Vicebio Ltd. to strengthen its respiratory vaccines portfolio. The acquisition would add Vicebio's early-stage RSV-hMPV combination vaccine candidate and its proprietary Molecular Clamp technology, which enabled the development of stable, liquid multivalent vaccines suitable for storage at 2-8 °C and administration via prefilled syringes.
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global vaccines market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive technical, market-oriented, and commercial study of the vaccines market. The secondary sources used for this study include World Health Organization (WHO), the Organization for Economic Co-operation and Development (OECD), National Center for Biotechnology Information (NCBI), Centers for Disease Control and Prevention (CDC), the Global Cancer Observatory (GLOBOCAN), the National Institutes of Health (NIH), Center of Disease Control & Prevention (CDC), US Department of Health and Human Services, National Institutes of Health (NIH), National Library of Medicine, National Center for Biotechnology Information (NCBI), National Institute of Allergy and Infectious Diseases (NIAID), World Cancer Research Fund International (WCRF International), European Medicines Agency (EMA), The National Medical Products Administration (NMPA), Global Alliance for Vaccines and Immunization (GAVI), United States Food & Drug Administration (US FDA), Orange book, Purple book, Clinical trials.gov, Pan American Health Organization (PAHO), United Nation International Children’s Emergency Fund (UNICEF), Department of health and Human Services (HHS), and International Society for Vaccines (ISV). Corporate filings include annual reports, SEC filings, investor presentations, and financial statements; research journals; press releases; and trade, business, and professional associations. Secondary data was collected and analyzed to arrive at the overall size of the global vaccines market, which was validated through primary research. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information.
Market Size Estimation
Both top-down and bottom-up approaches were employed to estimate and validate the overall size of the vaccines market. These methods were also widely used to determine the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Data Triangulation
After estimating the overall market size through the market size estimation process, the total market was divided into several segments and subsegments. Data triangulation and market breakdown techniques were used where applicable to finalize the overall market analysis and obtain precise statistics for all segments and subsegments. The data was triangulated by examining various factors and trends from both demand and supply sides.
Market Definition
A vaccine is a biologically formulated product designed to trigger active acquired immunity against a specific infectious or malignant disease. Vaccines work by stimulating the immune system to identify and fight harmful agents, such as viruses or bacteria. They generally consist of parts that resemble the disease-causing microorganism, often in weakened or deactivated forms, along with their toxins or surface proteins. The report solely focuses on human vaccines and does not include veterinary vaccines, which are outside the scope of this study.
Stakeholders
- Vaccine product manufacturers and suppliers
- Distributors and suppliers of vaccine products
- Vaccine research institutes
- Biotechnology and biopharmaceutical companies
- Contract manufacturing organizations (CMOs)
- Contract development and manufacturing organizations (CDMO)
- Suppliers and distributors of pharmaceutical products
- Research and development (R&D) companies
- Drug Manufacturers, Vendors, and Distributors
- Immunization centres
- Hospitals and laboratories
- Trade associations and industry bodies
- Regulatory bodies and government organizations
- Venture capitalists and investors
- Hospitals
- Specialty Clinics
Report Objectives
- To define, describe, and forecast the vaccines market by technology, type, disease indication, route of administration, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall vaccines market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to five regions: North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
- To profile the key players and analyze their market shares and core competencies2
- To track and analyze competitive developments, such as product launches, agreements, partnerships, acquisitions, regulatory approvals, and research & development activities
- To analyze and provide funding & investment activities, brand/product comparative analysis, and vendor valuation & financial metrics of the vaccines market.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Vaccines Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Europe Vaccines Market