
Europe Biomaterials Market Size, Growth, Share & Trends Analysis
Europe Biomaterials Market by Type (Metallic [Gold, Magnesium], Ceramic [Aluminum Oxide, Carbon], Polymer [Polyethylene, Polyester], Natural [Hyaluronic Acid, Collagen, Gelatin]), Application (Orthopedic, Dental, CVD, Ophthalmology) - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
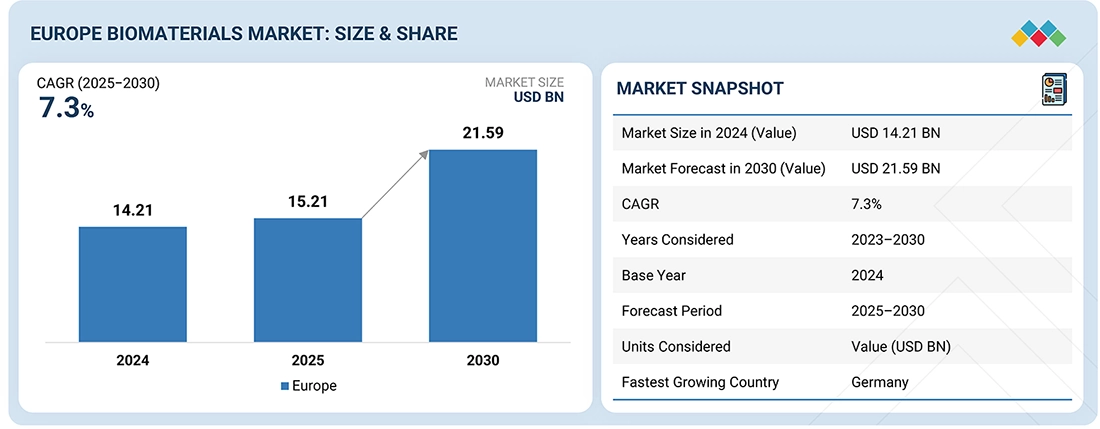
The Europe biomaterials market, valued at USD14.21 billion in 2024, stood at USD15.21 billion in 2025 and is projected to advance at a resilient CAGR of 7.3% from 2025 to 2030, culminating in a forecasted valuation of USD21.59 billion by the end of the period. Biomaterials are substances designed to interact with biological systems for medical purposes. These materials are designed to be compatible with living tissues and can be used in various medical applications, such as implants, prosthetics, drug delivery systems, and tissue engineering.
KEY TAKEAWAYS
-
BY REGIONGermany is the fastest-growing market for biomaterials in Europe, registering a CAGR 7.3% during the forecast period.
-
BY typeBy type, the metallic biomaterials segment is projected to register the highest CAGR of 7.6% during the forecast period.
-
BY ApplicationBy application, the orthopedic segment dominates the Europe biomaterials market.
-
COMPETITIVE LANDSCAPE - key playersBASF SE, Covestro AG, and DSM are identified as some of the star players in the Europe biomaterials market, given their strong market share and product footprint.
-
COMPETITIVE LANDSCAPE - Startups/SMEsSupraPolix BV and Cambrim GMBH Jellagen have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders
The Europe biomaterials market is expanding rapidly, driven by surging adoption of regenerative medicine therapies, 3D bioprinting innovations, and bioresorbable implants for orthopedics and tissue engineering. This is boosting demand for high-performance biomaterials compatible with stem cell scaffolds, drug-eluting stents, and personalized prosthetics.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The growth of the Europe biomaterials market is driven by the adoption of higher performing materials by hospitals and device manufacturers to support healthcare in general. More patients are being treated in Europe for cardiovascular diseases, musculoskeletal conditions, cancer, and neurological disorders. This raises the demand for safe and consistent biomaterials for the development of implants, scaffolds, and drug-delivery products. Healthcare in Europe revolves around the development of procedures that are effective in the long term and eliminate the need for reoperations. In addition to these, other biomaterials like bioresorbable polymers, bioactive ceramics, and materials based on collagen are being increasingly used. These assist in less invasive procedures, quick turnaround, and customized therapy. Stringent regulatory policies and cost-effective healthcare practices in Europe have encouraged the usage of biomaterials that can be manufactured in bulk and are safe for prolonged use.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing incidence of chronic conditions

-
Strong public healthcare systems and well-established medical device manufacturing base
Level
-
High development and clinical validation costs
Level
-
Rising demand for bioresorbable and natural biomaterials
Level
-
Ensuring long-term safety and performance of biomaterials
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Increasing incidence of chronic conditions
Increasing incidence of chronic and degenerative diseases, coupled with an aging population, is boosting demand for implantable and regenerative biomaterial solutions across Europe.
Restraint: High development and clinical validation costs
High development, clinical validation, and regulatory compliance costs under the EU Medical Device Regulation (MDR) are slowing product approvals and discouraging smaller innovators.
Opportunity: Rising demand for bioresorbable and natural biomaterials
Due to the increasing demand for bioresorbable and natural biomaterials supported by the EU, sustainability and circular bioeconomy initiatives are opening new growth opportunities in orthopedics, cardiovascular care, and tissue engineering.
Challenges : Ensuring long-term safety and performance of biomaterials
Ensuring the long-term safety, biocompatibility, and mechanical performance remains a significant challenge in the development of next-generation and nano-engineered biomaterials. Stringent MDR evidence requirements and concerns over adverse implant reaction is also a challenge.
EUROPE BIOMATERIALS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Medical-grade polyurethane foams, adhesives, and TPU films (Baymedix, Platilon, Dureflex) supplied to European wound-care and device OEMs for advanced dressings, wearable patches, and durable device housings | Enhances fluid management, comfort, and barrier protection | supports sterilizable single-use and reusable products | Enables soft, breathable, skin-friendly designs for long-wear medical and wearable devices |
 |
RESOMER bioresorbable polymers and other custom medical-grade excipients supplied across Europe for implantable orthopedic devices, 3D-printed implants, and long-acting parenteral drug-delivery systems | Offers precise control of mechanical strength and degradation, compatibility with terminal sterilization, and strong technical and CDMO support that accelerates development and approval of complex combination products |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe biomaterials industry is a strongly integrated community where material researchers, device makers, biotechnology and medical technology firms, research institutions, and physicians work together for the introduction of novel solutions based on biomaterials in medicine. Manufacturers, along with specialty material suppliers, offer high-performance polymers, metals, ceramics, and natural biomaterials. Meanwhile, contract research organizations, contract development and manufacturing organizations, and academic research facilities assist in preclinical studies, clinical studies, and commercialization efforts for implants, drug delivery systems, and regenerative medicine based on biomaterials. European medical facilities, reference, and reimbursement systems translate clinical confirmations for biomaterials into practical, innovative, and precision medicine for Europe on a daily basis through their normal operations.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
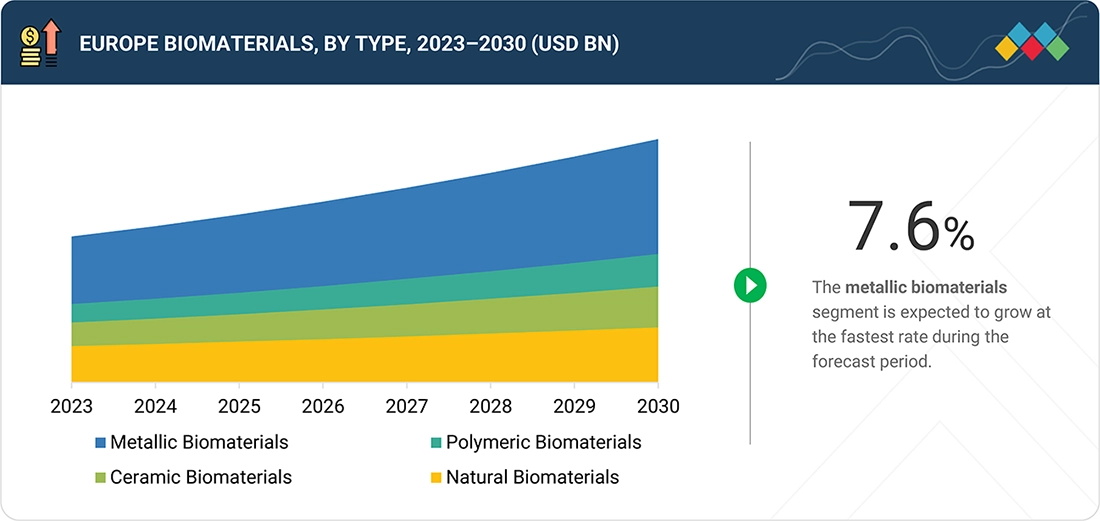
Europe Biomaterials Market, By Type
In 2024, the metallic biomaterials segment held a leading share in the Europe biomaterials market, supported by their extensive use in orthopedic, dental, and cardiovascular implants that require high mechanical strength and durability.
Europe Biomaterials Market, By Application
In 2024, the orthopedic segment had the largest share of the Europe biomaterials market, due to the growing burden of musculoskeletal disorders and rising volumes of joint replacement, spine, and fracture-repair procedures across the region.
REGION
Germany to be fastest-growing country in Europe biomaterials market during forecast period
Germany is the fastest-growing market for biomaterials in Europe. The market in the country is supported by integrated community where material researchers, device makers, biotechnology and medical technology firms, research institutions, and physicians work together for the introduction of novel solutions based on biomaterials in medicine. Manufacturers, along with specialty material suppliers, offer high-performance polymers, while contract research organizations, CROS, and academic research facilities assist in preclinical studies, clinical studies, and commercialization for implants, drug delivery systems, and regenerative medicine based on biomaterials.
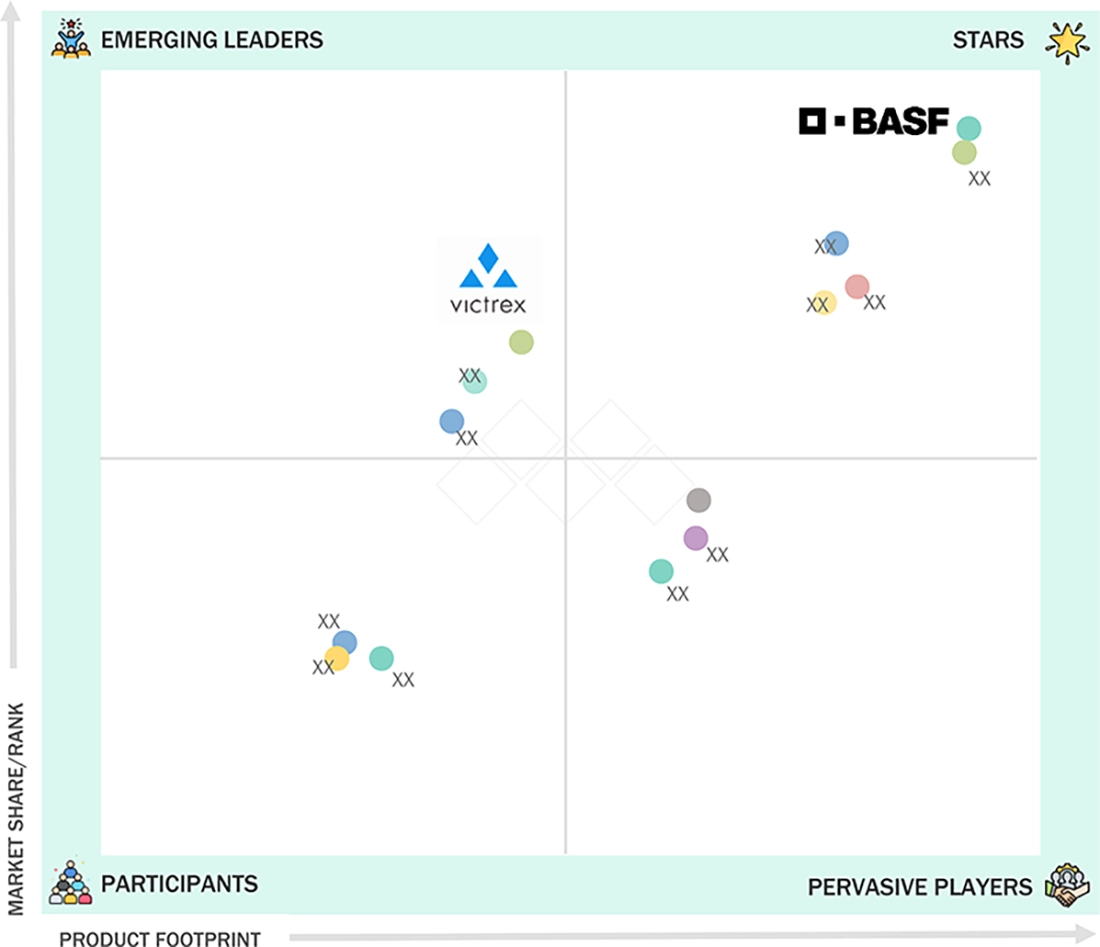
EUROPE BIOMATERIALS MARKET: COMPANY EVALUATION MATRIX
In the Europe biomaterials market, BASF (Star Player) has a broad portfolio of medical-grade plastics and superabsorbent polymers that European hospitals and device OEMs use in advanced wound-care dressings, surgical pads, drug-delivery components, and durable surgical instruments. Victrex Plc (Emerging Leader), through its Invibio medical PEEK business, is gaining presence in the market by supplying high-performance PEEK-OPTIMA polymers and composites for spinal fusion cages, fracture-fixation plates, joint-replacement components, and cranio-maxillofacial and dental implants across leading European orthopedic and neurosurgical centers.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- BASF SE (Germany)?
- Covestro AG (Germany)?
- DSM (Netherlands)?
- Corbion NV (Netherlands)?
- Evonik Industries AG (Germany)?
- Victrex PLC (UK)?
- CeramTec GmbH (Germany)?
- GELITA AG (Germany)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 14.21 Billion |
| Market Forecast in 2030 (Value) | USD 21.59 Billion |
| Growth Rate | CAGR of 7.3% from 2025-2030 |
| Years Considered | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Million) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, France, UK, Italy, Spain, Rest of Europe |
| Parent & Related Segment Reports |
Biomaterials Market Orthopedic Biomaterials Market North America Biomaterials Market Asia Pacific Biomaterials Market Polymeric Biomaterials Market |
WHAT IS IN IT FOR YOU: EUROPE BIOMATERIALS MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| To understand how European surgeons and hospitals choose biomaterials across orthopedic, dental, and cardiovascular procedures | Surveyed specialists and procurement teams to compare preferences for metallic, polymeric, ceramic, and bioresorbable materials by indication and country | Guides portfolio and messaging by revealing which biomaterials are gaining share in specific specialties and health systems |
| To Find Europe-based partners that can co-develop and manufacture advanced implants, coatings, and drug-delivery systems using GMP facilities | Profiled key European biomaterials CDMOs and medtech hubs, summarizing capabilities in polymer synthesis, 3D printing, sterilization, and regulatory support | Helps clients shortlist high-fit development and manufacturing partners in Europe, reducing time-to-market and investment risk for new biomaterial devices |
RECENT DEVELOPMENTS
- February 2025 : BASF launched HySorb B 6610 ZeroPCF, the first polyacrylate-based superabsorbent polymer marketed with a product carbon footprint of zero for hygiene and medical absorbent products.
Table of Contents

Methodology
This study involved four major activities in estimating the current size of the Europe biomaterials market. Exhaustive secondary research was carried out to collect information on the market, its peer markets, and its parent market. These findings, assumptions, and sizing were then validated with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate the market size of segments and subsegments.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive technical, market-oriented, and commercial study of the Europe biomaterials market. The secondary sources used for this study include the Journal of Biomedical Nanotechnology, Journal of Materials Science, Materials Science and Engineering, Journal of Biomedical Materials Research, Biomedical Engineering Society (BMES), Annual Reports, SEC Filings, Investor Presentations, Expert Interviews, and MarketsandMarkets Analysis. These sources were also used to obtain key information about major players, global product revenues, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives. Secondary data was collected and analyzed to arrive at the overall size of the Europe biomaterials market, which was validated through primary research.
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The market size of the Europe biomaterials market was estimated through multiple approaches. A detailed market estimation approach was followed to estimate and validate the value of the market and other dependent submarkets. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The major players in the industry and market have been identified through extensive primary and secondary research.
- The product and company revenues generated from the Europe biomaterials business of players operating in the market have been determined through secondary research and primary analysis.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Data Triangulation
After estimating the overall market size from the market size estimation process, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Biomaterials refer to substances that have been engineered to interact with biological systems for medical purposes. These materials are designed to be compatible with living tissues and can be used in various medical applications, such as implants, prosthetics, drug delivery systems, and tissue engineering. The primary goal of biomaterials is to enhance or replace the function of a body part, promote healing, and improve overall health. These materials are selected and engineered based on their biocompatibility, mechanical properties, and ability to integrate seamlessly with the biological environment. Biomaterials play a crucial role in advancing medical technologies and improving patient outcomes.
Key Stakeholders
- Biomaterial product manufacturing companies
- Healthcare service providers (including hospitals & specialty clinics)
- National and regional research boards and organizations
- Research & development companies
- Clinical research organizations (CROs)
- Healthcare technology companies
- Biotechnology companies
- Research laboratories & academic institutes
- Market research & consulting firms
- Regulatory bodies
The main objectives of this study are as follows:
- To define, describe, and forecast the Europe biomaterials market by type, application, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets with respect to individual growth trends, prospects, and contributions to the overall Europe biomaterials market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to Europe (Germany, France, the UK, Italy, Spain, and the Rest of Europe)
- To profile the key players and analyze their market shares and core competencies
- To track and analyze competitive developments, such as product launches, partnerships, agreements, collaborations, and expansions
- To benchmark players within the market using the proprietary “Company Evaluation Matrix” framework, which analyzes market players on various parameters within the broad categories of business and product excellence strategy
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report with additional efforts:
Product-Level Information
Country-wise Information:
- Analysis for additional countries (up to five)
Company Information:
- Detailed analysis and profiling of additional key market players across the globe
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Biomaterials Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Europe Biomaterials Market