
Nuclear Medicine Equipment Market: Growth, Size, Share, and Trends
Nuclear Medicine Equipment Market by Product (Imaging: PET, SPECT, Gamma; GM Counter, Dose Calibrator), Software (Image Management, PACS, Treatment, Analytic, Workflow), Therapy (Onco, Cardio, Neuro, Ortho), End User & Region - Global Forecasts to 2030




OVERVIEW
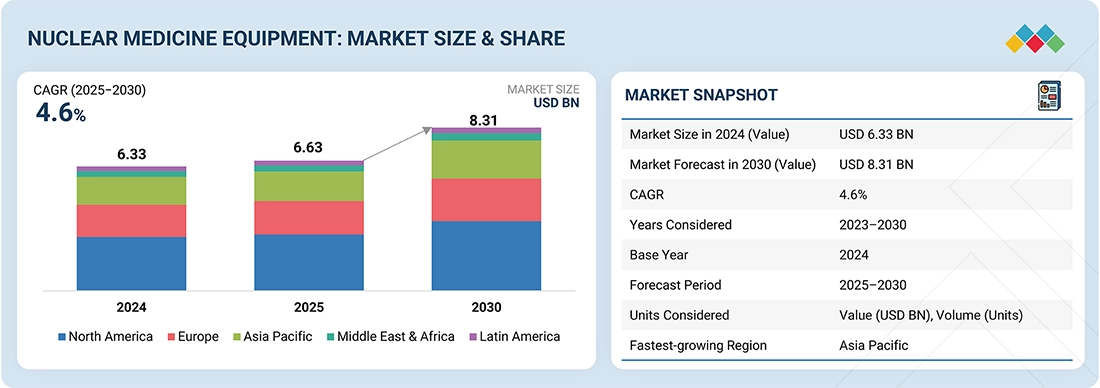
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The global nuclear medicine equipment market is projected to reach USD 8.31 billion by 2030, up from USD 6.63 billion in 2025, growing at a CAGR of 4.6% during the forecast period. The market growth is mainly driven by the rising incidence of cancer and cardiovascular diseases, increased demand for early and accurate diagnostic imaging, and greater adoption of hybrid imaging devices such as PET/CT and SPECT/CT.
KEY TAKEAWAYS
- North America is the largest market for nuclear medicine equipment with a market share of 43.3% in 2024.
- By system type, imaging modalities expected grow at the fastest CAGR of 4.7%.
- By therapeutic area, onology accounted for the largest market share in 2024.
- By dimension, 3D accounted for the largest market share in 2024.
- By end user, diagnostic imaging centers is expected to register the highest CAGR of 5.3%.
- The major market players have adopted both organic and inorganic strategies including partnerships and investments. For instance, GE HealthCare, Siemens Healthineers AG, Koninklijke Philips N.V., Mirion Technologies, Inc., and Canon Medical Systems Corporation entered into number of agreements and partnerships to cater the growing demand for advance nuclear medicine equipment.
- Trasis, Sofie, and Paire, among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders
The nuclear medicine equipment market continues to grow steadily, supported by government initiatives like the US National Cancer Institute's precision oncology programs and the EU's Euratom research projects. Additionally, advances in hybrid imaging technologies, along with strategic partnerships between manufacturers and healthcare providers, are improving diagnostic accuracy and treatment planning, fueling further innovation in the industry.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The impact on consumers' businesses stems from customer trends or disruptions. Hotbets are clients of nuclear medicine equipment manufacturers, and target applications are also clients of these manufacturers. Changes, which involve shifting trends or disruptions, will affect the revenues of end users. This revenue impact on end users will influence the revenue of hotbets, which in turn will affect the overall revenues of nuclear medicine equipment manufacturers.
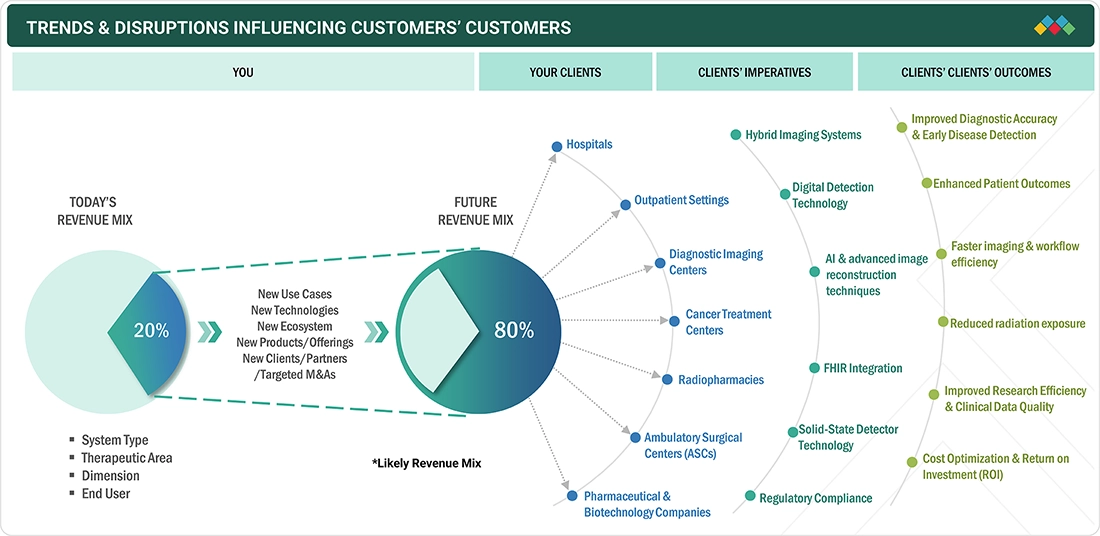
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rapid expansion of theranostics

-
Technological advancements in hybrid imaging modalities
Level
-
Capital-intensive nature of nuclear medicine equipment
-
Short half-life of radiopharmaceuticals
Level
-
Growing preference for personalized and precision medicines
-
Telemedicine and cloud-native platforms & mobile/remote imaging capabilities
Level
-
Global isotope supply chain instability
-
Cybersecurity and data privacy issues in imaging IT
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rapid expansion of theranostics
The rapid expansion of theranostics is a key driver of the nuclear medicine equipment market, integrating diagnostics and therapy through radiopharmaceuticals. Particularly in oncology, approaches like PSMA-based imaging and radioligand therapy are transforming prostate cancer and neuroendocrine tumor care, significantly improving detection rates and outcomes. Aligning with precision medicine, theranostics enables personalized, biomarker-guided treatment, with nearly half of FDA-approved oncology drugs since 1998 falling into this category. Therapies such as Lutetium-177 DOTATATE have shown marked survival benefits, while over 1,000 active clinical trials underscore growing adoption. Rising cancer incidence and demand for early, targeted intervention are fueling investments in PET/CT and SPECT/CT, cementing theranostics as a cornerstone of advanced nuclear medicine.
Restraint: Capital-intensive nature of nuclear medicine equipment
The capital-intensive nature of nuclear medicine equipment is a major obstacle, with PET/CT and SPECT systems costing up to USD 250,000 and total five-year operational expenses reaching USD 700,000. Such high costs limit adoption by smaller hospitals and create disparities in access. Procedure costs also vary widely, adding financial strain. However, this challenge is driving innovation in hybrid imaging, shared service models, and long-term service agreements to optimize utilization. Public-private collaborations are helping fund equipment in high-burden regions, while hospitals focus on asset management, cost-effective procurement, and financing strategies to balance affordability with access to advanced nuclear medicine.
Opportunity: Growing preference for personalized and precision medicines
The increasing demand for personalized and precision medicine creates a significant opportunity for the nuclear medicine equipment industry. By visualizing molecular and cellular processes unique to each patient, technologies like PET/CT and SPECT/CT enable precise disease characterization, therapy monitoring, and treatment adjustment. In oncology, these tools assist in identifying genetic mutations and guiding targeted, less toxic therapies. Progress in radiochemistry and imaging now expand beyond traditional applications to a broader range of conditions, supported by radiolabeled tracers that target specific pathways. With nearly 20 million nuclear medicine procedures performed annually in the US, ongoing discoveries of biomarkers and new radiotracers will further promote the adoption of precision medicine.
Challenge: Global isotope supply chain instability
Global isotope supply chain instability presents a major challenge for the nuclear medicine equipment market. Important isotopes like technetium-99m, used in about 85% of nuclear medicine scans (30 million annually), rely on a few aging reactors, making the supply highly vulnerable. Past crises, such as the 2009–2010 Tc-99m shortage, highlighted this fragility. The problem is worsened by reactor closures, limited production sites, geopolitical risks, and trade barriers. Since isotopes have short half-lives and cannot be stockpiled, even small disruptions can delay diagnostics and treatments, increase costs, and threaten patient care. This lack of redundancy emphasizes the urgent need for sustainable, diversified isotope production.
nuclear medicine equipment market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Al-enabled PET/CT and SPECT/CT platforms with advanced image reconstruction, theranostic support, and digital workflow solutions | Higher diagnostic accuracy, improved therapy monitoring, streamlined workflows, reduced scan times |
 |
Hybrid imaging systems integrating PET/MRI and PET/CT with cloud-based informatics, supporting personalized oncology and cardiology diagnostics | Enhanced precision in oncology/cardiology, data-driven decision support, better patient stratification |
 |
Advanced molecular imaging solutions with Al for lesion detection, radiomics, and dosimetry; strong focus on theranostics and therapy response tracking | Personalized treatment planning, accelerated clinical trials, improved therapy outcomes |
 |
Cost-effective PET/CT and SPECT systems with dose reduction technologies, integrated software for workflow automation, and accessibility in emerging markets | Expanded access to nuclear imaging, lower operational costs, improved patient safety and efficiency |
 |
Radiation detection and dosimetry solutions for nuclear medicine safety and compliance. | Improved radiation protection, regulatory compliance, and workflow efficiency. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The nuclear medicine equipment market ecosystem includes leading imaging system manufacturers such as GE HealthCare, Siemens Healthineers, Philips, and Canon, providing PET/CT, SPECT/CT, and hybrid modalities designed for precise diagnostics and theranostics. Radiopharmaceutical developers and cyclotron providers supply essential tracers, while AI and software partners improve image reconstruction, dosimetry, and workflow automation. End users such as hospitals, diagnostic imaging centers, and cancer treatment centers utilize these solutions for patient stratification, therapy monitoring, and personalized care. Regulators such as the FDA, EMA, and nuclear safety agencies ensure compliance, while government programs, research consortia, and private investments foster innovation and adoption. Collaboration across this ecosystem supports the growth of precision oncology and personalized medicine.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
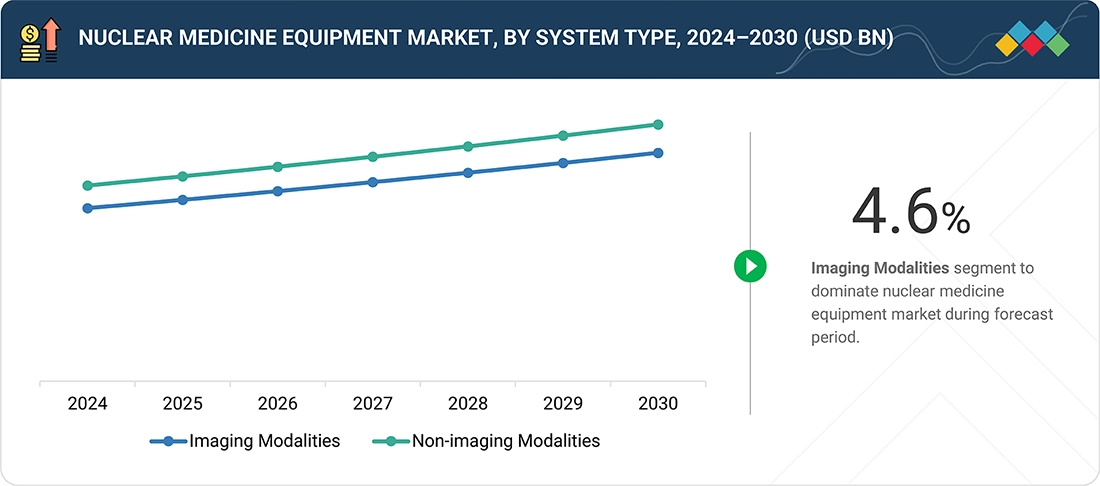
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Nuclear Medicine Equipment Market, By System Type
In 2024, the imaging modalities segment occupied the largest portion of the nuclear medicine equipment market. Widespread use of SPECT and PET systems allows for accurate diagnosis and monitoring in oncology, cardiology, and neurology. Growth is driven by advances in hybrid imaging, the development of targeted diagnostic solutions, and a preference for non-invasive, high-precision tools. The increasing number of nuclear medicine procedures and the global expansion of specialized facilities further boost this segment's growth. Additionally, rising investment in research and development of advanced imaging technologies is expected to support long-term market growth.
Nuclear Medicine Equipment Market, By Therapeutic Area
In 2024, the oncology segment held the largest share of the nuclear medicine equipment market. This growth is driven by the increasing global cancer burden, with over 20 million new cases in 2022, expected to reach 35 million by 2050. PET/CT and SPECT/CT imaging are essential for cancer detection, staging, treatment planning, and response assessment. The rising demand for radioligand therapies like ^177Lu-PSMA and ^177Lu-DOTATATE, advances in radiopharmaceuticals, regulatory support for theranostics, and improved reimbursement further propel this segment.
Nuclear Medicine Equipment Market, By Dimension
In 2024, the 3D segment held the largest share of the nuclear medicine equipment market. Technological advances, including time-of-flight (TOF) PET and iterative reconstruction techniques, are boosting the adoption of 3D nuclear medicine imaging. High-resolution 3D PET/CT and SPECT/CT systems enhance diagnostic accuracy, lesion visualization, and treatment planning in oncology, cardiology, and neurology. Increasing use of theranostic approaches further drives demand, enabling precise 3D quantification for dosimetry and therapy response monitoring, while also reducing scan times and improving image clarity.
REGION
North America to be fastest-growing region in global aerospace materials market during forecast period
In 2024, North America led the global nuclear medicine equipment market, driven by advanced healthcare infrastructure and widespread adoption of nuclear medicine procedures. The US alone performs nearly 20 million nuclear medicine procedures annually, with growing demand for hybrid PET/CT and SPECT/CT systems. The rise in chronic diseases, particularly cancer which is projected to cause over 2 million new cases and 612,000 deaths in the US by 2024 underscores the need for early and accurate diagnostic technologies. Key factors sustaining North America’s market dominance include favorable reimbursement policies, significant public and private R&D investment, and the presence of major players such as GE Healthcare, Siemens Healthineers, and Cardinal Health.
nuclear medicine equipment market: COMPANY EVALUATION MATRIX
In the nuclear medicine equipment market matrix, GE Healthcare (Star) leads with a strong market presence and an extensive product portfolio, fueled by its advanced imaging technologies, hybrid systems, and robust global service network. The company's focus on precision diagnostics, innovation in PET and SPECT systems, and integration with digital healthcare platforms solidifies its dominant position across hospitals, diagnostic centers, and research institutions. Spectrum Dynamics Medical (Emerging Leader) is gaining momentum with its innovative nuclear imaging solutions and compact, cost-effective systems, enhancing its position through niche offerings and patient-focused designs. While GE Healthcare continues to lead through scale, technological breadth, and international reach, Spectrum Dynamics Medical shows significant potential to move toward the Leaders' Quadrant as demand for efficient, high-resolution, and patient-centric nuclear medicine equipment increases.
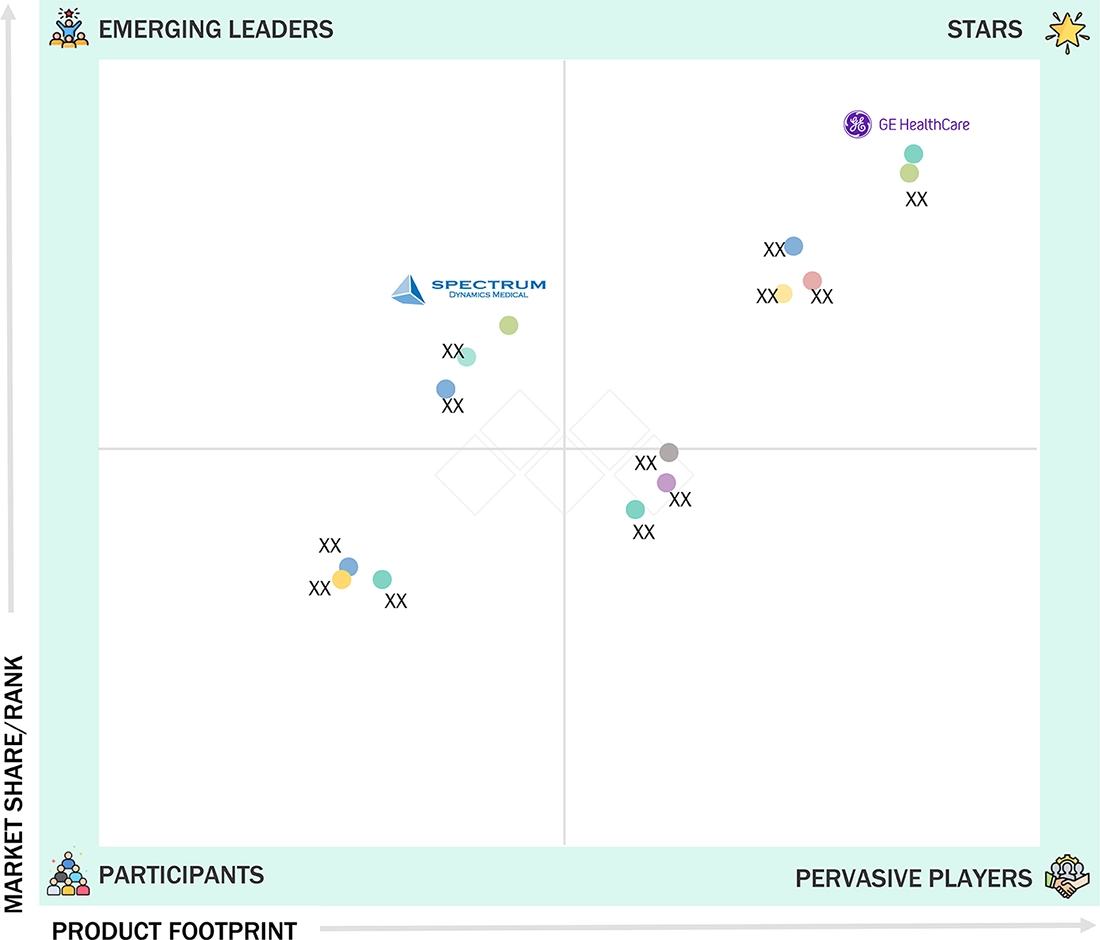
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- GE Healthcare
- Siemens Healthineers AG
- Koninklijke Philips N.V.
- Mirion Technologies, Inc.
- Canon Medical Systems Corporation
- Hermes Medical Solutions
- DOSIsoft SA
- Segami Corporation
- Winkgen Medical Systems GmBH & Co. KG
- Comecer S.P.A
- Syntermed Inc.
- UltraSPECT Inc.
- Lablogic Systems Ltd.
- Mediso Ltd.
- Catalyst MedTech
- Lemer Pax
- Spectrum Dynamics Medical
- Neusoft Medical Systems Co., Ltd.
- Brainlab SE
- Mirada Medical
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 6.33 Billion |
| Market Forecast in 2030 (value) | USD 8.31 Billion |
| Growth Rate | CAGR of 4.6% from 2025 to 2030 |
| Years Considered | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Billion), Volume (Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regions Covered | North America, Asia Pacific, Europe, Latin America, and Middle East & Africa |
WHAT IS IN IT FOR YOU: nuclear medicine equipment market REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Local Competitive Landscape | Profiles of key manufacturers (e.g., GE HealthCare, Siemens Healthineers, Philips), covering regional market share, segmental revenue (PET, SPECT, hybrid modalities), recent product launches, and integration of AI or theranostic capabilities. | Enables competitive benchmarking, identification of technology and portfolio gaps, and strategic planning for equipment investments. |
| Regional Market Entry Strategy | Regional or country-specific go-to-market analysis detailing barriers (regulatory, reimbursement, facility accreditation), demand drivers (oncology/cardiology disease burden, imaging volumes), and partnership opportunities (radiopharmaceutical supply, distributor networks). | Reduces entry risk for new market entrants, accelerates regional product adoption and supports localization strategies. |
| Local Risk & Opportunity Assessment | Identification of regulatory challenges, evolving safety standards, and untapped opportunities across hospitals, imaging centers, and outpatient clinics (e.g., adoption of hybrid PET/CT, SPECT/CT). | Supports proactive risk mitigation, strategic investments, and adaptation to evolving clinical practices and reimbursement models. |
| Technology Adoption by Region | Insights on regional adoption of advanced modalities (digital PET, time-of-flight, 3D imaging, dose management software) and drivers such as government funding, healthcare infrastructure, and R&D initiatives. | Guides R&D focus, product positioning, and investment decisions for targeted technology deployment and regional innovation partnerships. |
RECENT DEVELOPMENTS
- May 2025 : GE HealthCare obtained FDA 510(k) clearance for the Aurora dual-head SPECT/CT system and Clarify DL software. The system integrates functional and anatomical imaging for nuclear medicine, while the software enhances SPECT image reconstruction with deep learning technology.
- December 2024 : Siemens Healthineers acquired the Molecular Imaging business of Advanced Accelerator Applications (AAA), a network of diagnostic radiopharmaceuticals for positron emission tomography (PET) scans manufactured and distributed throughout Europe. This division includes software-integrated capabilities for PET radiopharmaceutical diagnostics, boosting Siemens's radio pharmacy supply and imaging infrastructure.
- December 2024 : Koninklijke Philips N.V. launched the CT 5300, an Al-powered CT Scanner that streamline workflows, reduces scan times, and improves diagnostic accuracy. It also collaborates with Annalise.ai to prioritize critical cases.
Table of Contents

Methodology
This market research study involved the extensive use of secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and financial study of the nuclear medicine equipment market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify critical qualitative and quantitative information and to assess market prospects. The size of the nuclear medicine equipment market was estimated through various secondary research approaches and triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
The secondary research process involved the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Some non-exclusive secondary sources include the World Health Organization (WHO), the Organisation for Economic Co-operation and Development (OECD), Healthcare Information and Management Systems Society (HIMSS), Centers for Disease Control and Prevention (CDC), ClinicalTrials.gov, Expert Interviews, and MarketsandMarkets Analysis.
Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the nuclear medicine equipment market. It was also used to obtain important information about the key players and market classification and segmentation according to industry trends, to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources are mainly industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, technology developers, researchers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess prospects.
Primary research was conducted to identify segmentation types, industry trends, key players, and key market dynamics such as drivers, restraints, opportunities, challenges, and strategies adopted by key players.
After completing the market engineering process, which includes calculations for market statistics, market breakdown, size estimations, forecasting, and data triangulation, extensive primary research was conducted. This research aimed to gather information and verify the critical numbers obtained during the market analysis. Additionally, primary research was conducted to identify different types of market segmentation, analyze industry trends, evaluate the competitive landscape of nuclear medicine equipment offered by various players, and understand key market dynamics such as drivers, restraints, opportunities, challenges, industry trends, and strategies employed by key market participants.
In the complete market engineering process, the top-down and bottom-up approaches and several data triangulation methods were extensively used to perform the market estimation and market forecasting for the overall market segments and subsegments listed in this report. Extensive qualitative and quantitative analysis was performed on the complete market engineering process to list the key information/insights throughout the report.
Breakdown of Primary Interviews
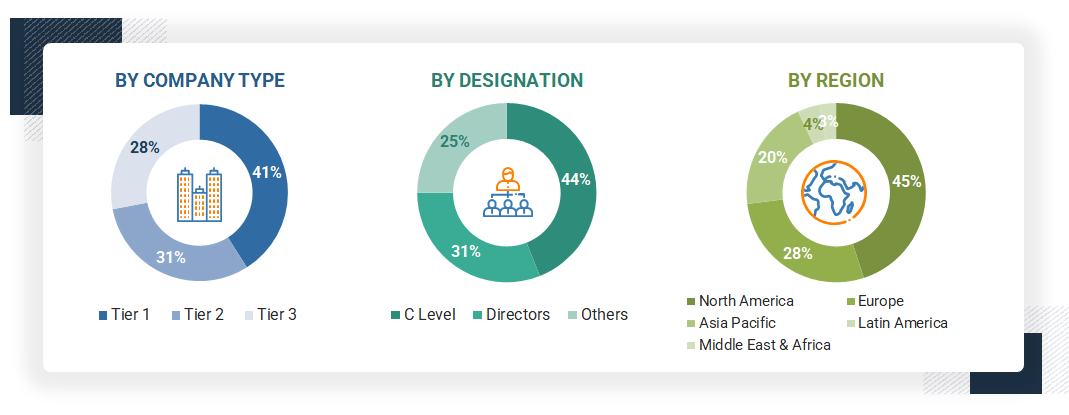
Note 1: Other designations include sales, marketing, and product managers.
Note 2: Tiers are defined based on a company’s total revenue, as of 2024: Tier 1 = >USD 1 billion, Tier 2 = USD 500 million to USD 1 billion, and Tier 3 = < USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The market size estimates and forecasts provided in this study are derived through a mix of the bottom-up approach (revenue share analysis of leading players) and top-down approach (assessment of utilization/adoption/penetration trends, by product type, therapeutic area, dimension, end user, and region).
Data Triangulation
After arriving at the overall market size, from the market size estimation process explained above, the nuclear medicine equipment market was split into segments and subsegments. To complete the overall market engineering process and to arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the nuclear medicine equipment market.
Market Definition
Nuclear medicine equipment refers to specialized medical devices used to perform diagnostic and therapeutic procedures involving radioactive substances. These include imaging systems such as PET (Positron Emission Tomography) and SPECT (Single Photon Emission Computed Tomography) scanners, as well as gamma cameras and hybrid imaging systems. The equipment enables the detection, imaging, and tracking of radiopharmaceuticals within the body, playing a crucial role in disease diagnosis, monitoring, and targeted radionuclide therapy.
Stakeholders
- Nuclear Medicine Equipment Vendors & Imaging Device Manufacturers
- Healthcare Providers (Hospitals, Outpatient Settings, Diagnostics & Imaging Centers, Radiopharmacies, Other Healthcare Providers)
- Clinical Research Organizations (CROs)
- Research & Development (R&D) Companies
- Business Research & Consulting Service Providers
- Medical Research Laboratories
- Academic Medical Centers/Universities/Hospitals/Research Institutes
- Regulatory Bodies (FDA, EMA, IAEA)
- AI & Algorithm Developers
- Venture Capitalists
- Advocacy Groups
- Investors & Financial Institutions
- Contract Manufacturing Organizations (CMOs)
- Contract Research Organizations (CROs)
Report Objectives
- To define, describe, and forecast the nuclear medicine equipment market by product type, therapeutic area, dimension, end user, and region
- To provide detailed information regarding the major drivers, restraints, opportunities, and challenges influencing market growth
- To strategically analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall nuclear medicine equipment market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To forecast the size of the nuclear medicine equipment market in five main regions (and their respective countries): North America, Europe, Asia Pacific, the Middle East & Africa, and Latin America
- To provide key industry insights, such as supply chain, regulatory, patent, and recession impact analysis
- To profile the key players in the market and comprehensively analyze their core competencies
- To track and analyze competitive developments, such as product launches & upgrades, collaborations, partnerships, acquisitions, investments, contracts, agreements, alliances, mergers, funding, and expansions of the leading players in the market
- To benchmark players within the market using the company evaluation matrix, which analyzes market players on various parameters within the broad categories of business strategy, market share, and product offering
Key Questions Addressed by the Report
Which are the top industry players in the nuclear medicine equipment market?
Prominent players include Hermes Medical Solutions (Sweden), DOSIsoft (France), Segami Corporation (US), GE HealthCare (US), Siemens Healthineers AG (Germany), Koninklijke Philips N.V. (Netherlands), Mirion Technologies, Inc. (US), and Comecer S.p.A (Italy).
Which segments have been included in the nuclear medicine equipment market report?
The nuclear medicine equipment market report includes segments based on product type, therapeutic area, dimension, end user, and region.
Which geographical region is dominating the nuclear medicine equipment market?
North America is the dominant region in the nuclear medicine equipment market, while Asia Pacific is projected to experience the fastest growth during the forecast period.
Which end-user segments have been included in the nuclear medicine equipment market report?
The end-user segments in the report include hospitals, diagnostic imaging centers, cancer treatment centers, and other end users.
What is the total CAGR expected to be recorded for the nuclear medicine equipment market during 2025–2030?
The nuclear medicine equipment market is expected to grow at a CAGR of 4.62% from 2025 to 2030.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Nuclear Medicine Equipment Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Nuclear Medicine Equipment Market