This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, key market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the particle therapy market. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in product therapy markets. The primary sources from the demand side include medical OEMs, Analytical instrument OEMs, CDMOs, and service providers, among others. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends & key market dynamics.
A breakdown of the primary respondents is provided below:
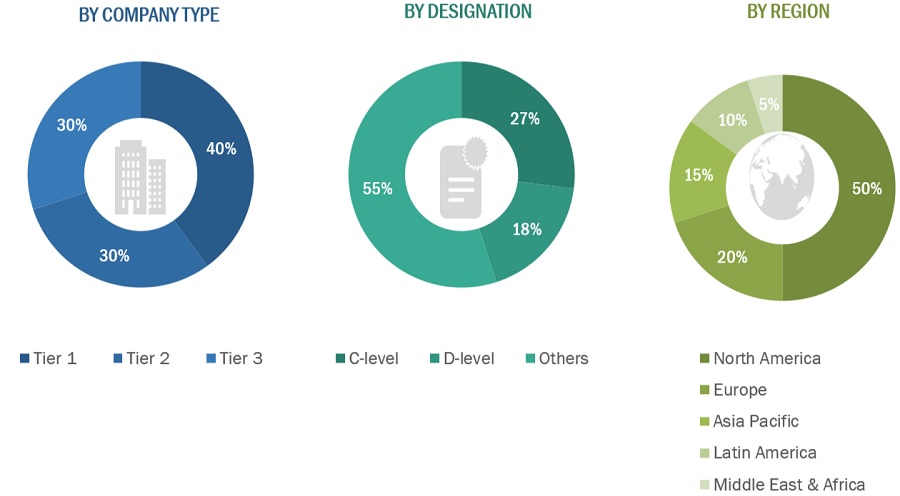
*Others include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
Note: Companies are classified into tiers based on their total revenue. As of 2021, Tier 1 = >USD 2 billion, Tier 2 = USD 50 million to USD 2 billion, and Tier 3 = <USD 50 million.
To know about the assumptions considered for the study, download the pdf brochure
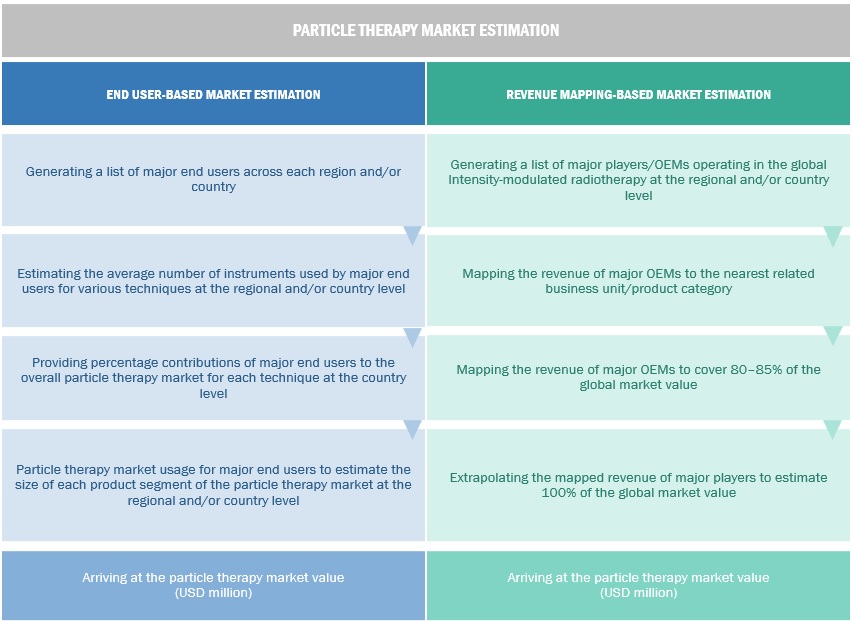
Market Estimation Methodology
In this report, the global particle therapy market size was arrived at by using the revenue share analysis of leading players. For this purpose, key players in the market were identified, and their revenues from the particle therapy market business were determined through various insights gathered during the primary and secondary research phases. Secondary research included the study of the annual and financial reports of the top market players. In contrast, primary research included extensive interviews with key opinion leaders, such as CEOs, directors, and key marketing executives.
To calculate the global market value, segmental revenues were calculated based on the revenue mapping of major solution/service providers. This process involved the following steps:
-
Generating a list of major global players operating in the particle therapy market.
-
Mapping annual revenues generated by major global players from the global particle therapy market (or nearest reported business unit/product category)
-
Revenue mapping of key players to cover a major share of the global market, as of 2022
-
Extrapolating the global value of the particle therapy market
To know about the assumptions considered for the study, Request for Free Sample Report
Data Triangulation
After arriving at the overall market size from the market size estimation process explained above, the global particle therapy market was split into segments and subsegments. Data triangulation and market breakdown procedures were employed to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data was triangulated by studying various factors and trends from both the demand and supply sides. Additionally, the particle therapy market was validated using both top-down and bottom-up approaches.
Market Definition
Particle therapy is an advanced modality of external beam radiotherapy that uses positively charged particles such as protons or heavy ions for cancer treatment. Particle therapy offers a more precise and effective mode of radiation, which accurately targets tumors and minimizes damage to healthy tissues.
Key Stakeholders
-
Radiotherapy product manufacturers
-
Distributors, suppliers, and commercial service providers
-
Healthcare service providers
-
Clinical research organizations (CROs)
-
Radiotherapy service providers
-
Radiotherapy product distributors
-
Medical research laboratories
-
Cancer care centers
-
Cancer research organizations
-
Academic medical centers and universities
-
Market research and consulting firms
Objectives of the Study
-
To define, describe, and forecast the particle therapy market based on product,type, system, application, cancer type and region.
-
To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
-
To strategically analyze the micro markets with respect to individual growth trends, prospects, and contributions to the total market.
-
To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders.
-
To profile the key market players and comprehensively analyze their market shares and core competencies.
-
To forecast the revenue of the market segments with respect to five main regions, namely, North America (US and Canada), Europe (Germany, France, the UK, Italy, Spain, and Rest of Europe), the Asia Pacific (China, Japan, India, South Korea, Australia, and Rest of Asia Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East & Africa.
-
To track and analyze competitive developments such as new product launches and approvals; agreements, partnerships, expansions, acquisitions; and collaborations in the particle therapy market.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the present global particle therapy market
Product Analysis
-
Product matrix, which gives a detailed comparison of the product portfolios of the top thirteen companies.
Company Information
-
Detailed analysis and profiling of additional market players (up to 13)
Geographic Analysis
-
Further breakdown of the Rest of Europe's particle therapy market into Russia, Belgium, the Netherlands, Switzerland, Austria, Finland, Sweden, Poland, and Portugal among other
-
Further breakdown of the Rest of Asia Pacific particle therapy market into Singapore, Taiwan, New Zealand, Philippines, Malaysia, and other APAC countries
-
Further breakdown of the Rest of the world particle therapy market into Latin America, MEA, and Africa



Amy
Mar, 2022
Which is the targeted audience in the study of Particle Therapy Market?.
Angela
Mar, 2022
How the growing adoption of particle therapy in emerging markets is benefiting global growth of Particle Therapy Market?.
Shirley
Mar, 2022
What are the recent developments in the global Particle Therapy Market?.